You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000144_02359
You are here: Home > Sequence: MGYG000000144_02359
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Barnesiella intestinihominis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Barnesiellaceae; Barnesiella; Barnesiella intestinihominis | |||||||||||
| CAZyme ID | MGYG000000144_02359 | |||||||||||
| CAZy Family | GH97 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 282855; End: 285353 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH97 | 29 | 603 | 5.5e-138 | 0.9730586370839936 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam10566 | Glyco_hydro_97 | 1.19e-55 | 273 | 510 | 3 | 278 | Glycoside hydrolase 97. This domain is the catalytic region of the bacterial glycosyl-hydrolase family 97. This central part of the GH97 family protein sequences represents a typical and complete (beta/alpha)8-barrel or catalytic TIM-barrel type domain. The N- and C-terminal parts of the sequences, mainly consisting of beta-strands, form two additional non-catalytic domains. In all known glycosidases with the (beta-alpha)8-barrel fold, the amino acid residues at the active site are located on the C-termini of the beta-strands. |
| pfam14508 | GH97_N | 2.75e-40 | 32 | 265 | 1 | 234 | Glycosyl-hydrolase 97 N-terminal. This N-terminal domain of glycosyl-hydrolase-97 contributes part of the active site pocket. It is also important for contact with the catalytic and C-terminal domains of the whole. |
| pfam14509 | GH97_C | 1.62e-25 | 513 | 605 | 1 | 97 | Glycosyl-hydrolase 97 C-terminal, oligomerization. Glycosyl-hydrolase-97 is made up of three tightly linked and highly conserved globular domains. The C-terminal domain is found to be necessary for oligomerization of the whole molecule in order to create the active-site pocket and the Ca++-binding site. |
| cd04081 | CBM35_galactosidase-like | 1.70e-17 | 617 | 744 | 2 | 125 | Carbohydrate Binding Module family 35 (CBM35); appended mainly to enzymes that bind alpha-D-galactose (CBM35-Gal), including glycoside hydrolase (GH) families GH27 and GH43. This family includes carbohydrate binding module family 35 (CBM35); these are non-catalytic carbohydrate binding domains that are appended mainly to enzymes that bind alpha-D-galactose (CBM35-Gal), including glycoside hydrolase (GH) families GH27 and GH43. Examples of proteins which contain CBM35s belonging to this family includes the CBM35 of an exo-beta-1,3-galactanase from Phanerochaete chrysosporium 9 (Pc1,3Gal43A) which is appended to a GH43 domain, and the CBM35 domain of two bifunctional proteins with beta-L-arabinopyranosidase/alpha-D-galactopyranosidase activities from Fusarium oxysporum 12S, Foap1 and Foap2 (Fo/AP1 and Fo/AP2), that are appended to GH27 domains. CBM35s are unique in that they display conserved specificity through extensive sequence similarity but divergent function through their appended catalytic modules. They are known to bind alpha-D-galactose (Gal), mannan (Man), xylan, glucuronic acid (GlcA), a beta-polymer of mannose, and possibly glucans, forming four subfamilies based on general ligand specificities (galacto, urono, manno, and gluco configurations). Some CBM35s bind their ligands in a calcium-dependent manner. In contrast to most CBMs that are generally rigid proteins, CBM35 undergoes significant conformational change upon ligand binding. GH43 includes beta-xylosidases and beta-xylanases, using aryl-glycosides as substrates, while family GH27 includes alpha-galactosidases, alpha-N-acetylgalactosaminidases, and isomaltodextranases. |
| cd02795 | CBM6-CBM35-CBM36_like | 1.11e-06 | 617 | 744 | 1 | 124 | Carbohydrate Binding Module 6 (CBM6) and CBM35_like superfamily. Carbohydrate binding module family 6 (CBM6, family 6 CBM), also known as cellulose binding domain family VI (CBD VI), and related CBMs (CBM35 and CBM36). These are non-catalytic carbohydrate binding domains found in a range of enzymes that display activities against a diverse range of carbohydrate targets, including mannan, xylan, beta-glucans, cellulose, agarose, and arabinans. These domains facilitate the strong binding of the appended catalytic modules to their dedicated, insoluble substrates. Many of these CBMs are associated with glycoside hydrolase (GH) domains. CBM6 is an unusual CBM as it represents a chimera of two distinct binding sites with different modes of binding: binding site I within the loop regions and binding site II on the concave face of the beta-sandwich fold. CBM36s are calcium-dependent xylan binding domains. CBM35s display conserved specificity through extensive sequence similarity, but divergent function through their appended catalytic modules. This alignment model also contains the C-terminal domains of bacterial insecticidal toxins, where they may be involved in determining insect specificity through carbohydrate binding functionality. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ATB36826.1 | 2.99e-120 | 32 | 602 | 29 | 587 |
| AEE97714.1 | 2.66e-117 | 32 | 608 | 16 | 594 |
| ADO69318.1 | 1.05e-116 | 47 | 602 | 47 | 589 |
| AEC02290.1 | 2.58e-109 | 30 | 605 | 49 | 625 |
| AWS47373.1 | 3.82e-105 | 47 | 766 | 63 | 774 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5XFM_A | 7.61e-51 | 33 | 599 | 22 | 637 | Crystalstructure of beta-arabinopyranosidase [Bacteroides thetaiotaomicron],5XFM_B Crystal structure of beta-arabinopyranosidase [Bacteroides thetaiotaomicron],5XFM_C Crystal structure of beta-arabinopyranosidase [Bacteroides thetaiotaomicron],5XFM_D Crystal structure of beta-arabinopyranosidase [Bacteroides thetaiotaomicron] |
| 3A24_A | 1.88e-50 | 32 | 570 | 6 | 603 | Crystalstructure of BT1871 retaining glycosidase [Bacteroides thetaiotaomicron],3A24_B Crystal structure of BT1871 retaining glycosidase [Bacteroides thetaiotaomicron] |
| 5E1Q_A | 1.93e-49 | 32 | 570 | 20 | 617 | Mutant(D415G) GH97 alpha-galactosidase in complex with Gal-Lac [Bacteroides thetaiotaomicron VPI-5482],5E1Q_B Mutant (D415G) GH97 alpha-galactosidase in complex with Gal-Lac [Bacteroides thetaiotaomicron VPI-5482] |
| 5HQC_A | 5.12e-38 | 32 | 604 | 5 | 658 | AGlycoside Hydrolase Family 97 enzyme R171K variant from Pseudoalteromonas sp. strain K8 [Pseudoalteromonas sp. K8] |
| 5HQ4_A | 6.85e-38 | 32 | 604 | 5 | 658 | AGlycoside Hydrolase Family 97 enzyme from Pseudoalteromonas sp. strain K8 [Pseudoalteromonas sp. K8],5HQA_A A Glycoside Hydrolase Family 97 enzyme in complex with Acarbose from Pseudoalteromonas sp. strain K8 [Pseudoalteromonas sp. K8] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| D7CFN7 | 1.41e-70 | 25 | 603 | 36 | 619 | Probable retaining alpha-galactosidase OS=Streptomyces bingchenggensis (strain BCW-1) OX=749414 GN=SBI_01652 PE=3 SV=1 |
| Q8A6L0 | 1.38e-49 | 32 | 570 | 27 | 624 | Retaining alpha-galactosidase OS=Bacteroides thetaiotaomicron (strain ATCC 29148 / DSM 2079 / JCM 5827 / CCUG 10774 / NCTC 10582 / VPI-5482 / E50) OX=226186 GN=BT_1871 PE=1 SV=1 |
| G8JZS4 | 5.14e-26 | 89 | 602 | 107 | 721 | Glucan 1,4-alpha-glucosidase SusB OS=Bacteroides thetaiotaomicron (strain ATCC 29148 / DSM 2079 / JCM 5827 / CCUG 10774 / NCTC 10582 / VPI-5482 / E50) OX=226186 GN=susB PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
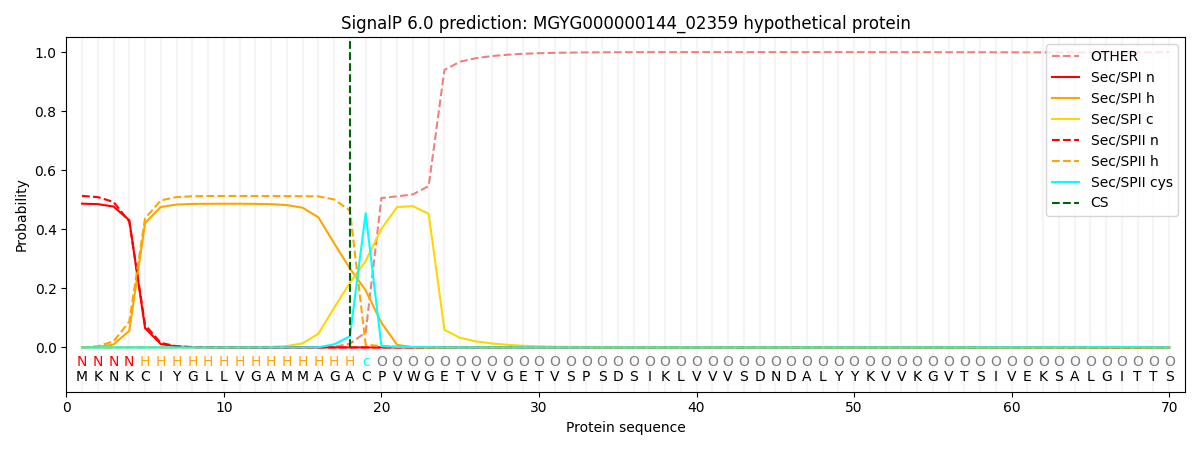
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000421 | 0.479688 | 0.519298 | 0.000218 | 0.000196 | 0.000161 |
