You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001035_00001
You are here: Home > Sequence: MGYG000001035_00001
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Pseudomonas_E putida_P | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Proteobacteria; Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae; Pseudomonas_E; Pseudomonas_E putida_P | |||||||||||
| CAZyme ID | MGYG000001035_00001 | |||||||||||
| CAZy Family | GT4 | |||||||||||
| CAZyme Description | Glutamate/aspartate import solute-binding protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 855; End: 1772 Strand: - | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| PRK10797 | PRK10797 | 2.72e-141 | 21 | 301 | 20 | 302 | glutamate and aspartate transporter subunit; Provisional |
| cd13688 | PBP2_GltI_DEBP | 2.46e-102 | 30 | 269 | 1 | 238 | Substrate-binding domain of ABC aspartate-glutamate transporter; the type 2 periplasmic binding protein fold. This subfamily represents the periplasmic-binding protein component of ABC transporter specific for carboxylic amino acids, including GtlI from Escherichia coli. The aspartate-glutamate binding domain belongs to the type 2 periplasmic binding protein fold superfamily (PBP2), whose many members are involved in chemotaxis and uptake of nutrients and other small molecules from the extracellular space as a primary receptor. The PBP2 proteins are typically comprised of two globular subdomains connected by a flexible hinge and bind their ligand in the cleft between these domains in a manner resembling a Venus flytrap. After binding their specific ligand with high affinity, they can interact with a cognate membrane transport complex comprised of two integral membrane domains and two receptor cytoplasmically-located ATPase domains. This interaction triggers the ligand translocation across the cytoplasmic membrane energized by ATP hydrolysis. |
| cd01000 | PBP2_Cys_DEBP_like | 1.49e-63 | 30 | 269 | 1 | 228 | Substrate-binding domain of cysteine- and aspartate/glutamate-binding proteins; the type 2 periplasmic-binding protein fold. This family comprises of the periplasmic-binding protein component of ABC transporters specific for cysteine and carboxylic amino acids, as well as their closely related proteins. The cysteine and aspartate-glutamate binding domains belong to the type 2 periplasmic binding protein fold superfamily (PBP2), whose many members are involved in chemotaxis and uptake of nutrients and other small molecules from the extracellular space as a primary receptor. The PBP2 proteins are typically comprised of two globular subdomains connected by a flexible hinge and bind their ligand in the cleft between these domains in a manner resembling a Venus flytrap. After binding their specific ligand with high affinity, they can interact with a cognate membrane transport complex comprised of two integral membrane domains and two receptor cytoplasmically-located ATPase domains. This interaction triggers the ligand translocation across the cytoplasmic membrane energized by ATP hydrolysis. |
| cd13689 | PBP2_BsGlnH | 4.30e-62 | 30 | 269 | 1 | 228 | Substrate binding domain of ABC glutamine transporter from Bacillus subtilis; the type 2 periplasmic-bindig protein fold. This group includes periplasmic glutamine-binding domain GlnP from Bacillus subtilis and its related proteins. The GlnP domain belongs to the type 2 periplasmic binding protein fold superfamily (PBP2), whose many members are involved in chemotaxis and uptake of nutrients and other small molecules from the extracellular space as a primary receptor. The PBP2 proteins are typically comprised of two globular subdomains connected by a flexible hinge and bind their ligand in the cleft between these domains in a manner resembling a Venus flytrap. After binding their specific ligand with high affinity, they can interact with a cognate membrane transport complex comprised of two integral membrane domains and two receptor cytoplasmically-located ATPase domains. This interaction triggers the ligand translocation across the cytoplasmic membrane energized by ATP hydrolysis. |
| pfam00497 | SBP_bac_3 | 7.14e-58 | 39 | 269 | 1 | 223 | Bacterial extracellular solute-binding proteins, family 3. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBV05439.1 | 4.35e-116 | 23 | 299 | 21 | 295 |
| QIF58808.1 | 4.35e-116 | 23 | 299 | 21 | 295 |
| AWS50894.1 | 4.35e-116 | 23 | 299 | 21 | 295 |
| QZY63525.1 | 4.35e-116 | 23 | 299 | 21 | 295 |
| BBV13529.1 | 4.35e-116 | 23 | 299 | 21 | 295 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2VHA_A | 7.16e-109 | 29 | 299 | 9 | 277 | DEBP[Shigella flexneri],2VHA_B DEBP [Shigella flexneri] |
| 2IA4_A | 4.15e-103 | 29 | 299 | 9 | 277 | Crystalstructure of Novel amino acid binding protein from Shigella flexneri [Shigella flexneri 2a str. 301],2IA4_B Crystal structure of Novel amino acid binding protein from Shigella flexneri [Shigella flexneri 2a str. 301] |
| 5EYF_A | 1.07e-22 | 61 | 269 | 40 | 236 | CrystalStructure of Solute-binding Protein from Enterococcus faecium with Bound Glutamate [Enterococcus faecium DO],5EYF_B Crystal Structure of Solute-binding Protein from Enterococcus faecium with Bound Glutamate [Enterococcus faecium DO] |
| 2V25_A | 1.64e-14 | 11 | 268 | 14 | 257 | Structureof the Campylobacter jejuni antigen Peb1A, an aspartate and glutamate receptor with bound aspartate [Campylobacter jejuni],2V25_B Structure of the Campylobacter jejuni antigen Peb1A, an aspartate and glutamate receptor with bound aspartate [Campylobacter jejuni] |
| 2PYY_A | 1.78e-13 | 48 | 269 | 12 | 221 | ChainA, Ionotropic glutamate receptor bacterial homologue [Nostoc punctiforme PCC 73102],2PYY_B Chain B, Ionotropic glutamate receptor bacterial homologue [Nostoc punctiforme PCC 73102],2PYY_C Chain C, Ionotropic glutamate receptor bacterial homologue [Nostoc punctiforme PCC 73102] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q9I402 | 8.86e-156 | 1 | 302 | 1 | 301 | L-glutamate/L-aspartate-binding protein OS=Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) OX=208964 GN=PA1342 PE=1 SV=1 |
| Q9ZF60 | 5.28e-107 | 29 | 299 | 32 | 300 | Glutamate/aspartate import solute-binding protein OS=Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) OX=99287 GN=gltI PE=3 SV=3 |
| P37902 | 3.03e-106 | 29 | 299 | 32 | 300 | Glutamate/aspartate import solute-binding protein OS=Escherichia coli (strain K12) OX=83333 GN=gltI PE=1 SV=2 |
| O34563 | 1.02e-23 | 24 | 271 | 33 | 269 | ABC transporter glutamine-binding protein GlnH OS=Bacillus subtilis (strain 168) OX=224308 GN=glnH PE=2 SV=1 |
| P27676 | 1.41e-18 | 27 | 248 | 49 | 259 | Glutamine-binding protein OS=Geobacillus stearothermophilus OX=1422 GN=glnH PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
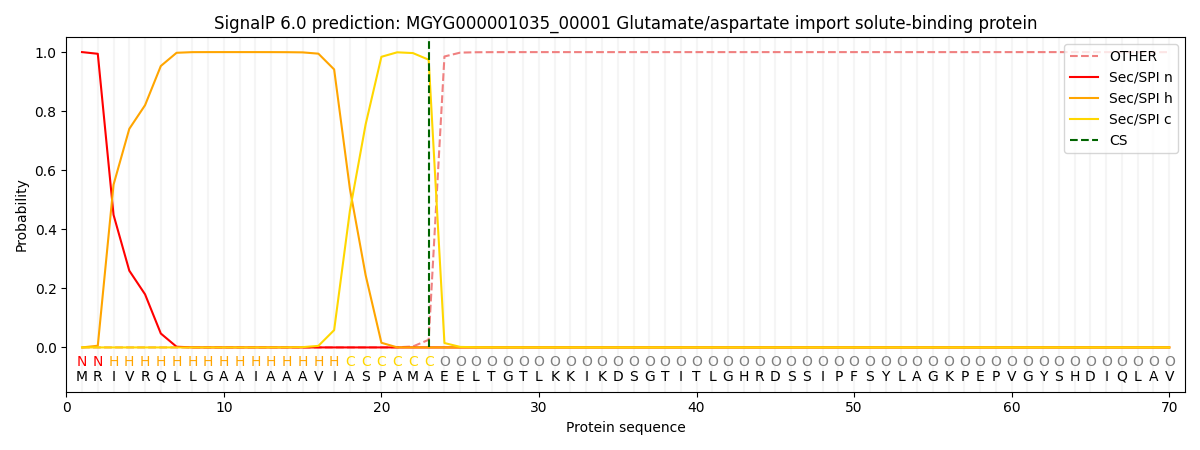
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000520 | 0.998555 | 0.000246 | 0.000244 | 0.000229 | 0.000190 |
