You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001078_00990
You are here: Home > Sequence: MGYG000001078_00990
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Rothia sp001808955 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Actinobacteriota; Actinomycetia; Actinomycetales; Micrococcaceae; Rothia; Rothia sp001808955 | |||||||||||
| CAZyme ID | MGYG000001078_00990 | |||||||||||
| CAZy Family | CBM13 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 10803; End: 12743 Strand: - | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd07496 | Peptidases_S8_13 | 1.35e-122 | 223 | 501 | 1 | 285 | Peptidase S8 family domain, uncharacterized subfamily 13. This family is a member of the Peptidases S8 or Subtilases serine endo- and exo-peptidase clan. They have an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. The stability of subtilases may be enhanced by calcium, some members have been shown to bind up to 4 ions via binding sites with different affinity. Some members of this clan contain disulfide bonds. These enzymes can be intra- and extracellular, some function at extreme temperatures and pH values. |
| cd07484 | Peptidases_S8_Thermitase_like | 6.39e-72 | 194 | 503 | 3 | 257 | Peptidase S8 family domain in Thermitase-like proteins. Thermitase is a non-specific, trypsin-related serine protease with a very high specific activity. It contains a subtilisin like domain. The tertiary structure of thermitase is similar to that of subtilisin BPN'. It contains a Asp/His/Ser catalytic triad. Members of the peptidases S8 (subtilisin and kexin) and S53 (sedolisin) clan include endopeptidases and exopeptidases. The S8 family has an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. Serine acts as a nucleophile, aspartate as an electrophile, and histidine as a base. The S53 family contains a catalytic triad Glu/Asp/Ser with an additional acidic residue Asp in the oxyanion hole, similar to that of subtilisin. The serine residue here is the nucleophilic equivalent of the serine residue in the S8 family, while glutamic acid has the same role here as the histidine base. However, the aspartic acid residue that acts as an electrophile is quite different. In S53 the it follows glutamic acid, while in S8 it precedes histidine. The stability of these enzymes may be enhanced by calcium, some members have been shown to bind up to 4 ions via binding sites with different affinity. There is a great diversity in the characteristics of their members: some contain disulfide bonds, some are intracellular while others are extracellular, some function at extreme temperatures, and others at high or low pH values. |
| cd07477 | Peptidases_S8_Subtilisin_subset | 1.94e-66 | 223 | 501 | 1 | 229 | Peptidase S8 family domain in Subtilisin proteins. This group is composed of many different subtilisins: Pro-TK-subtilisin, subtilisin Carlsberg, serine protease Pb92 subtilisin, and BPN subtilisins just to name a few. Pro-TK-subtilisin is a serine protease from the hyperthermophilic archaeon Thermococcus kodakaraensis and consists of a signal peptide, a propeptide, and a mature domain. TK-subtilisin is matured from pro-TK-subtilisin upon autoprocessing and degradation of the propeptide. Unlike other subtilisins though, the folding of the unprocessed form of pro-TK-subtilisin is induced by Ca2+ binding which is almost completed prior to autoprocessing. Ca2+ is required for activity unlike the bacterial subtilisins. The propeptide is not required for folding of the mature domain unlike the bacterial subtilases because of the stability produced from Ca2+ binding. Subtilisin Carlsberg is extremely similar in structure to subtilisin BPN'/Novo thought it has a 30% difference in amino acid sequence. The substrate binding regions are also similar and 2 possible Ca2+ binding sites have been identified recently. Subtilisin Carlsberg possesses the highest commercial importance as a proteolytic additive for detergents. Serine protease Pb92, the serine protease from the alkalophilic Bacillus strain PB92, also contains two calcium ions and the overall folding of the polypeptide chain closely resembles that of the subtilisins. Members of the peptidases S8 and S35 clan include endopeptidases, exopeptidases and also a tripeptidyl-peptidase. The S8 family has an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. The S53 family contains a catalytic triad Glu/Asp/Ser. The stability of these enzymes may be enhanced by calcium, some members have been shown to bind up to 4 ions via binding sites with different affinity. Some members of this clan contain disulfide bonds. These enzymes can be intra- and extracellular, some function at extreme temperatures and pH values. |
| cd07473 | Peptidases_S8_Subtilisin_like | 1.17e-65 | 222 | 502 | 2 | 258 | Peptidase S8 family domain in Subtilisin-like proteins. This family is a member of the Peptidases S8 or Subtilases serine endo- and exo-peptidase clan. They have an Asp/His/Ser catalytic triad similar to that found in trypsin-like proteases, but do not share their three-dimensional structure and are not homologous to trypsin. The stability of subtilases may be enhanced by calcium, some members have been shown to bind up to 4 ions via binding sites with different affinity. Some members of this clan contain disulfide bonds. These enzymes can be intra- and extracellular, some function at extreme temperatures and pH values. |
| pfam00082 | Peptidase_S8 | 2.42e-61 | 221 | 503 | 1 | 273 | Subtilase family. Subtilases are a family of serine proteases. They appear to have independently and convergently evolved an Asp/Ser/His catalytic triad, like that found in the trypsin serine proteases (see pfam00089). Structure is an alpha/beta fold containing a 7-stranded parallel beta sheet, order 2314567. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AIY26234.1 | 1.71e-106 | 194 | 528 | 129 | 468 |
| QZY84418.1 | 2.50e-105 | 73 | 498 | 67 | 450 |
| CAG23448.1 | 1.52e-100 | 132 | 524 | 76 | 465 |
| ALN86161.1 | 1.93e-99 | 136 | 528 | 94 | 479 |
| ATE72186.1 | 2.70e-99 | 136 | 528 | 94 | 479 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5YL7_A | 2.97e-109 | 194 | 520 | 4 | 332 | Proteasesfrom Pseudoalteromonas arctica PAMC 21717 (Pro21717) [Pseudoalteromonas arctica] |
| 3LPC_A | 8.95e-92 | 194 | 526 | 3 | 338 | Crystalstructure of a subtilisin-like protease [Dichelobacter nodosus] |
| 3LPA_A | 8.95e-92 | 194 | 526 | 3 | 338 | Crystalstructure of a subtilisin-like protease [Dichelobacter nodosus VCS1703A] |
| 3TI7_A | 5.13e-91 | 194 | 530 | 4 | 343 | Crystalstructure of the basic protease BprV from the ovine footrot pathogen, Dichelobacter nodosus [Dichelobacter nodosus VCS1703A] |
| 3TI9_A | 5.13e-91 | 194 | 530 | 4 | 343 | Crystalstructure of the basic protease BprB from the ovine footrot pathogen, Dichelobacter nodosus [Dichelobacter nodosus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P23314 | 9.44e-107 | 136 | 523 | 90 | 465 | Extracellular protease OS=Xanthomonas campestris pv. campestris (strain ATCC 33913 / DSM 3586 / NCPPB 528 / LMG 568 / P 25) OX=190485 GN=XCC0851 PE=3 SV=1 |
| P42780 | 1.05e-92 | 135 | 526 | 83 | 471 | Extracellular subtilisin-like protease OS=Dichelobacter nodosus OX=870 PE=3 SV=1 |
| P42779 | 1.04e-90 | 127 | 533 | 77 | 478 | Extracellular basic protease OS=Dichelobacter nodosus OX=870 GN=bprV PE=1 SV=1 |
| Q99405 | 8.58e-42 | 221 | 524 | 134 | 380 | M-protease OS=Alkalihalobacillus clausii (strain KSM-K16) OX=66692 GN=aprE PE=1 SV=2 |
| P29600 | 1.93e-41 | 221 | 524 | 23 | 269 | Subtilisin Savinase OS=Lederbergia lentus OX=1467 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
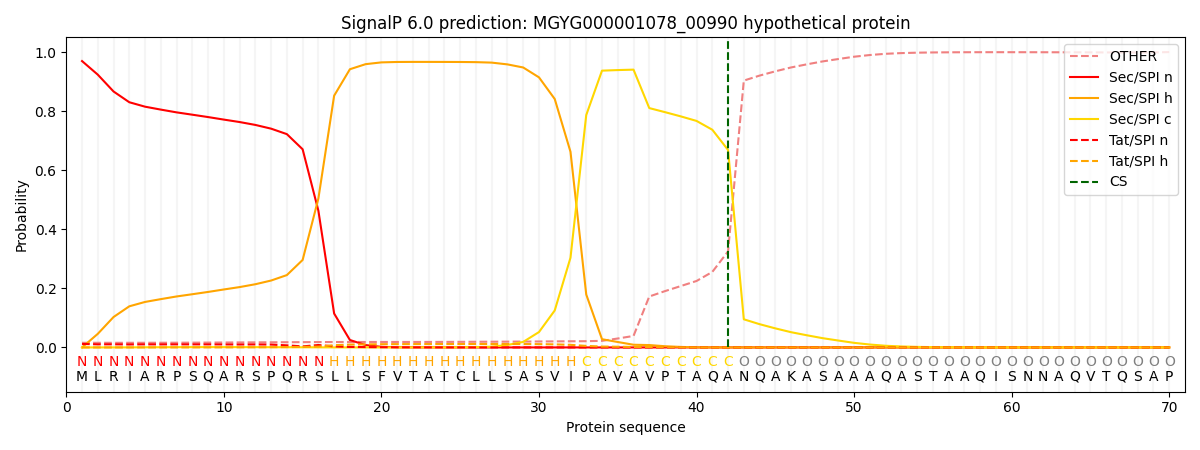
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.017579 | 0.965250 | 0.003172 | 0.012901 | 0.000770 | 0.000266 |
