You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001657_01288
You are here: Home > Sequence: MGYG000001657_01288
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | RC9 sp900760425 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; UBA932; RC9; RC9 sp900760425 | |||||||||||
| CAZyme ID | MGYG000001657_01288 | |||||||||||
| CAZy Family | GH18 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 7271; End: 9211 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06542 | GH18_EndoS-like | 4.61e-37 | 378 | 613 | 28 | 255 | Endo-beta-N-acetylglucosaminidases are bacterial chitinases that hydrolyze the chitin core of various asparagine (N)-linked glycans and glycoproteins. The endo-beta-N-acetylglucosaminidases have a glycosyl hydrolase family 18 (GH18) catalytic domain. Some members also have an additional C-terminal glycosyl hydrolase family 20 (GH20) domain while others have an N-terminal domain of unknown function (pfam08522). Members of this family include endo-beta-N-acetylglucosaminidase S (EndoS) from Streptococcus pyogenes, EndoF1, EndoF2, EndoF3, and EndoH from Flavobacterium meningosepticum, and EndoE from Enterococcus faecalis. EndoS is a secreted endoglycosidase from Streptococcus pyogenes that specifically hydrolyzes the glycan on human IgG between two core N-acetylglucosamine residues. EndoE is a secreted endoglycosidase, encoded by the ndoE gene in Enterococcus faecalis, that hydrolyzes the glycan on human RNase B. |
| pfam08522 | DUF1735 | 3.04e-13 | 230 | 326 | 25 | 118 | Domain of unknown function (DUF1735). This domain of unknown function is found in a number of bacterial proteins including acylhydrolases. The structure of this domain has a beta-sandwich fold. |
| cd14948 | BACON | 4.14e-10 | 31 | 113 | 1 | 83 | Bacteroidetes-Associated Carbohydrate-binding (putative) Often N-terminal (BACON) domain. The BACON domain is found in diverse domain architectures and accociated with a wide variety of domains, including carbohydrate-active enzymes and proteases. It was named for its suggested function of carbohydrate binding; the latter was inferred from domain architectures, sequence conservation, and phyletic distribution. However, recent experimental data suggest that its primary function in Bacteroides ovatus endo-xyloglucanase BoGH5A is to distance the catalytic module from the cell surface and confer additional mobility to the catalytic domain for attack of the polysaccharide. No evidence for a direct role in carbohydrate binding could be found in that case. The large majority of BACON domains are found in Bacteroidetes. |
| cd14948 | BACON | 7.37e-10 | 121 | 202 | 1 | 83 | Bacteroidetes-Associated Carbohydrate-binding (putative) Often N-terminal (BACON) domain. The BACON domain is found in diverse domain architectures and accociated with a wide variety of domains, including carbohydrate-active enzymes and proteases. It was named for its suggested function of carbohydrate binding; the latter was inferred from domain architectures, sequence conservation, and phyletic distribution. However, recent experimental data suggest that its primary function in Bacteroides ovatus endo-xyloglucanase BoGH5A is to distance the catalytic module from the cell surface and confer additional mobility to the catalytic domain for attack of the polysaccharide. No evidence for a direct role in carbohydrate binding could be found in that case. The large majority of BACON domains are found in Bacteroidetes. |
| pfam13004 | BACON | 1.13e-06 | 61 | 112 | 3 | 60 | Putative binding domain, N-terminal. The BACON (Bacteroidetes-Associated Carbohydrate-binding Often N-terminal) domain is an all-beta domain found in diverse architectures, principally in combination with carbohydrate-active enzymes and proteases. These architectures suggest a carbohydrate-binding function which is also supported by the nature of BACON's few conserved amino-acids. The phyletic distribution of BACON and other data tentatively suggest that it may frequently function to bind mucin. Further work with the characterized structure of a member of glycoside hydrolase family 5 enzyme, Structure 3ZMR, has found no evidence for carbohydrate-binding for this domain. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBL11372.1 | 8.30e-151 | 19 | 643 | 15 | 648 |
| BBL08580.1 | 8.30e-151 | 19 | 643 | 15 | 648 |
| CBK65046.1 | 2.17e-146 | 30 | 643 | 22 | 642 |
| QRQ55675.1 | 8.77e-81 | 213 | 627 | 50 | 465 |
| QDH54393.1 | 8.77e-81 | 213 | 627 | 50 | 465 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6T8I_A | 2.31e-69 | 211 | 644 | 23 | 447 | Crystalstructure of wild type EndoBT-3987 from Bacteroides thetaiotamicron VPI-5482 [Bacteroides thetaiotaomicron VPI-5482],6T8K_A Crystal structure of Bacteroides thetaiotamicron EndoBT-3987 in complex with Man9GlcNAc product in P1 [Bacteroides thetaiotaomicron VPI-5482],6T8K_B Crystal structure of Bacteroides thetaiotamicron EndoBT-3987 in complex with Man9GlcNAc product in P1 [Bacteroides thetaiotaomicron VPI-5482],6T8L_A Crystal structure of Bacteroides thetaiotamicron EndoBT-3987 with Man9GlcNAc product in P212121 [Bacteroides thetaiotaomicron VPI-5482],6TCW_A Crystal structure of Bacteroides thetaiotamicron EndoBT-3987 with Man5GlcNAc product [Bacteroides thetaiotaomicron VPI-5482],7NWF_A Chain A, Endo-beta-N-acetylglucosaminidase F1 [Bacteroides thetaiotaomicron VPI-5482] |
| 6TCV_B | 4.79e-67 | 211 | 644 | 23 | 447 | Crystalstructure of Bacteroides thetaiotamicron EndoBT-3987 in complex with Man9GlcNAc2Asn substrate [Bacteroides thetaiotaomicron VPI-5482] |
| 3POH_A | 5.03e-65 | 211 | 644 | 23 | 447 | Crystalstructure of an endo-beta-N-acetylglucosaminidase (BT_3987) from BACTEROIDES THETAIOTAOMICRON VPI-5482 at 1.55 A resolution [Bacteroides thetaiotaomicron VPI-5482] |
| 2EBN_A | 3.93e-48 | 347 | 627 | 7 | 287 | CRYSTALSTRUCTURE OF ENDO-BETA-N-ACETYLGLUCOSAMINIDASE F1, AN ALPHA(SLASH)BETA-BARREL ENZYME ADAPTED FOR A COMPLEX SUBSTRATE [Elizabethkingia meningoseptica] |
| 1EDT_A | 5.22e-27 | 347 | 594 | 6 | 245 | ChainA, ENDO-BETA-N-ACETYLGLUCOSAMINIDASE H, ENDO H [Streptomyces plicatus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P36911 | 2.19e-47 | 331 | 627 | 43 | 337 | Endo-beta-N-acetylglucosaminidase F1 OS=Elizabethkingia meningoseptica OX=238 GN=endOF1 PE=1 SV=1 |
| P04067 | 6.74e-26 | 347 | 594 | 48 | 287 | Endo-beta-N-acetylglucosaminidase H OS=Streptomyces plicatus OX=1922 PE=1 SV=1 |
| P80036 | 1.71e-25 | 352 | 592 | 57 | 289 | Endo-beta-N-acetylglucosaminidase OS=Flavobacterium sp. (strain SK1022) OX=148444 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
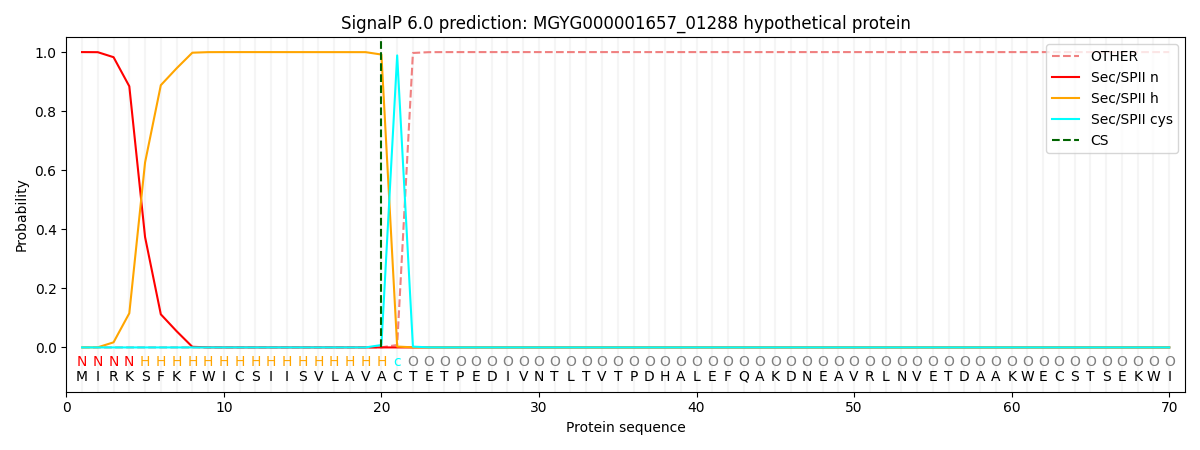
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000004 | 1.000047 | 0.000000 | 0.000000 | 0.000000 |
