You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001920_01578
You are here: Home > Sequence: MGYG000001920_01578
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phocaeicola sp900546355 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Phocaeicola; Phocaeicola sp900546355 | |||||||||||
| CAZyme ID | MGYG000001920_01578 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | Arabinoxylan arabinofuranohydrolase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 17116; End: 18471 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 21 | 304 | 8.5e-111 | 0.9964028776978417 |
| CBM6 | 327 | 450 | 8.4e-37 | 0.8695652173913043 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd18618 | GH43_Xsa43E-like | 2.98e-153 | 28 | 306 | 1 | 275 | Glycosyl hydrolase family 43, including Butyrivibrio proteoclasticus arabinofuranosidase Xsa43E. This glycosyl hydrolase family 43 (GH43) subgroup belongs to the GH43_AXH-like subgroup which includes enzymes that have been characterized with beta-xylosidase (EC 3.2.1.37), alpha-L-arabinofuranosidase (EC 3.2.1.55), alpha-1,2-L-arabinofuranosidase 43A (arabinan-specific; EC 3.2.1.-), endo-alpha-L-arabinanase as well as arabinoxylan arabinofuranohydrolase (AXH) activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. AXHs specifically hydrolyze the glycosidic bond between arabinofuranosyl substituents and xylopyranosyl backbone residues of arabinoxylan. This subgroup includes Cellvibrio japonicus arabinan-specific alpha-1,2-arabinofuranosidase, CjAbf43A, which confers its specificity by a surface cleft that is complementary to the helical backbone of the polysaccharide, and Butyrivibrio proteoclasticus GH43 enzyme Xsa43E, also an arabinofuranosidase, which has been shown to cleave arabinose side chains from short segments of xylan. Several of these enzymes also contain carbohydrate binding modules (CBMs) that bind cellulose or xylan. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd08990 | GH43_AXH_like | 1.71e-90 | 30 | 305 | 1 | 268 | Glycosyl hydrolase family 43 protein, includes arabinoxylan arabinofuranohydrolase, beta-xylosidase, endo-1,4-beta-xylanase, and alpha-L-arabinofuranosidase. This subgroup includes Bacillus subtilis arabinoxylan arabinofuranohydrolase (XynD;BsAXH-m23;BSU18160), Butyrivibrio proteoclasticus alpha-L-arabinofuranosidase (Xsa43E;bpr_I2319), Clostridium stercorarium alpha-L-arabinofuranosidase XylA, and metagenomic beta-xylosidase (EC 3.2.1.37) / alpha-L-arabinofuranosidase (EC 3.2.1.55) CoXyl43. It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. The GH43_AXH-like subgroup includes enzymes that have been characterized with beta-xylosidase, alpha-L-arabinofuranosidase, endo-alpha-L-arabinanase as well as arabinoxylan arabinofuranohydrolase (AXH) activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. AXHs specifically hydrolyze the glycosidic bond between arabinofuranosyl substituents and xylopyranosyl backbone residues of arabinoxylan. Metagenomic beta-xylosidase/alpha-L-arabinofuranosidase CoXyl43 shows synergy with Trichoderma reesei cellulases and promotes plant biomass saccharification by degrading xylo-oligosaccharides, such as xylobiose and xylotriose, into the monosaccharide xylose. Studies show that the hydrolytic activity of CoXyl43 is stimulated in the presence of calcium. Several of these enzymes also contain carbohydrate binding modules (CBMs) that bind cellulose or xylan. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd09003 | GH43_XynD-like | 4.30e-63 | 21 | 307 | 1 | 315 | Glycosyl hydrolase family 43 protein such as Bacillus subtilis arabinoxylan arabinofuranohydrolase (XynD;BsAXH-m23;BSU18160). This glycosyl hydrolase family 43 (GH43) subgroup includes characterized Bacillus subtilis arabinoxylan arabinofuranohydrolase (AXH), Caldicellulosiruptor sp. Tok7B.1 beta-1,4-xylanase (EC 3.2.1.8) / alpha-L-arabinosidase (EC 3.2.1.55) XynA, Caldicellulosiruptor sp. Rt69B.1 xylanase C (EC 3.2.1.8) XynC, and Caldicellulosiruptor saccharolyticus beta-xylosidase (EC 3.2.1.37)/ alpha-L-arabinofuranosidase (EC 3.2.1.55) XynF. It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. It belongs to the GH43_AXH-like subgroup which includes enzymes that have been annotated as having beta-xylosidase, alpha-L-arabinofuranosidase and arabinoxylan alpha-L-1,3-arabinofuranohydrolase, xylanase (endo-alpha-L-arabinanase) as well as AXH activities. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. AXHs specifically hydrolyze the glycosidic bond between arabinofuranosyl substituents and xylopyranosyl backbone residues of arabinoxylan. Bacillus subtilis AXH (BsAXH-m2,3) has been shown to cleave arabinose units from O-2- or O-3-mono-substituted xylose residues and superposition of its structure with known structures of the GH43 exo-acting enzymes, beta-xylosidase and alpha-L-arabinanase, each in complex with their substrate, reveals a different orientation of the sugar backbone. Several of these enzymes also contain carbohydrate binding modules (CBMs) that bind cellulose or xylan. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd09004 | GH43_bXyl-like | 6.11e-61 | 30 | 304 | 1 | 263 | Glycosyl hydrolase family 43 protein such as Bacteroides thetaiotaomicron VPI-5482 alpha-L-arabinofuranosidases (BT3675;BT_3675) and (BT3662;BT_3662); includes mostly xylanases. This glycosyl hydrolase family 43 (GH43) subgroup includes enzymes that have been annotated as xylan-digesting beta-xylosidase (EC 3.2.1.37) and xylanase (endo-alpha-L-arabinanase, EC 3.2.1.8) activities, as well the Bacteroides thetaiotaomicron VPI-5482 alpha-L-arabinofuranosidases (EC 3.2.1.55) (BT3675;BT_3675) and (BT3662;BT_3662). It belongs to the glycosyl hydrolase clan F (according to carbohydrate-active enzymes database (CAZY)) which includes family 43 (GH43) and 62 (GH62) families. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd04084 | CBM6_xylanase-like | 1.73e-53 | 326 | 449 | 2 | 123 | Carbohydrate Binding Module 6 (CBM6); many are appended to glycoside hydrolase (GH) family 11 and GH43 xylanase domains. This family includes carbohydrate binding module 6 (CBM6) domains that are appended mainly to glycoside hydrolase (GH) family domains, including GH3, GH11, and GH43 domains. These CBM6s are non-catalytic carbohydrate binding domains that facilitate the strong binding of the GH catalytic modules with their dedicated, insoluble substrates. Examples of proteins having CMB6s belonging to this family are Microbispora bispora GghA, a 1,4-beta-D-glucan glucohydrolase (GH3); Clostridium thermocellum xylanase U (GH11), and Penicillium purpurogenum ABF3, a bifunctional alpha-L-arabinofuranosidase/xylobiohydrolase (GH43). GH3 comprises enzymes with activities including beta-glucosidase (hydrolyzes beta-galactosidase) and beta-xylosidase (hydrolyzes 1,4-beta-D-xylosidase). GH11 family comprises enzymes with xylanase (endo-1,4-beta-xylanase) activity which catalyze the hydrolysis of beta-1,4 bonds of xylan, the major component of hemicelluloses, to generate xylooligosaccharides and xylose. GH43 includes beta-xylosidases and beta-xylanases, using aryl-glycosides as substrates. CBM6 is an unusual CBM as it represents a chimera of two distinct binding sites with different modes of binding: binding site I within the loop regions and binding site II on the concave face of the beta-sandwich fold. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT89600.1 | 2.03e-247 | 1 | 450 | 2 | 451 |
| QRQ50185.1 | 3.16e-247 | 1 | 449 | 1 | 448 |
| ALJ59342.1 | 1.17e-246 | 1 | 450 | 2 | 451 |
| QUT43848.1 | 1.82e-246 | 1 | 449 | 1 | 448 |
| QIU93952.1 | 1.63e-156 | 10 | 450 | 15 | 460 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3QEE_A | 2.28e-89 | 18 | 314 | 1 | 293 | Thestructure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107],3QEE_B The structure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107] |
| 3QEF_A | 3.63e-88 | 18 | 314 | 1 | 293 | Thestructure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107],3QEF_B The structure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107] |
| 3QED_A | 7.18e-84 | 18 | 314 | 8 | 300 | Thestructure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107],3QED_B The structure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107],3QED_C The structure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107],3QED_D The structure and function of an arabinan-specific alpha-1,2-arabinofuranosidase identified from screening the activities of bacterial GH43 glycoside hydrolases [Cellvibrio japonicus Ueda107] |
| 4NOV_A | 2.20e-75 | 20 | 311 | 45 | 339 | Xsa43E,a GH43 family enzyme from Butyrivibrio proteoclasticus [Butyrivibrio proteoclasticus B316] |
| 3C7E_A | 2.25e-62 | 21 | 449 | 14 | 484 | Crystalstructure of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from Bacillus subtilis. [Bacillus subtilis],3C7F_A Crystal structure of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from bacillus subtilis in complex with xylotriose. [Bacillus subtilis],3C7H_A Crystal structure of glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from Bacillus subtilis in complex with AXOS-4-0.5. [Bacillus subtilis],3C7O_A Crystal structure of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase from Bacillus subtilis in complex with cellotetraose. [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P45796 | 4.27e-64 | 21 | 449 | 39 | 507 | Arabinoxylan arabinofuranohydrolase OS=Paenibacillus polymyxa OX=1406 GN=xynD PE=1 SV=1 |
| Q45071 | 2.19e-61 | 21 | 449 | 40 | 510 | Arabinoxylan arabinofuranohydrolase OS=Bacillus subtilis (strain 168) OX=224308 GN=xynD PE=1 SV=2 |
| P49943 | 1.37e-28 | 28 | 311 | 13 | 324 | Xylosidase/arabinosidase OS=Bacteroides ovatus OX=28116 GN=xsa PE=2 SV=1 |
| P48791 | 6.09e-18 | 23 | 304 | 6 | 315 | Beta-xylosidase OS=Prevotella ruminicola OX=839 GN=xynB PE=3 SV=1 |
| P10478 | 3.55e-14 | 343 | 449 | 322 | 419 | Endo-1,4-beta-xylanase Z OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=xynZ PE=1 SV=3 |
SignalP and Lipop Annotations help
This protein is predicted as SP
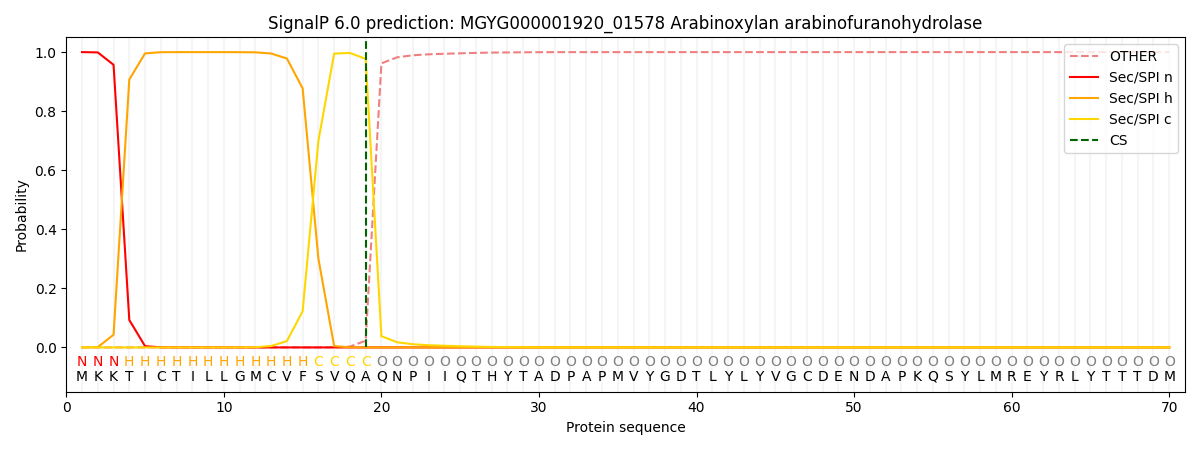
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000395 | 0.998763 | 0.000258 | 0.000192 | 0.000190 | 0.000172 |
