You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002024_00444
You are here: Home > Sequence: MGYG000002024_00444
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
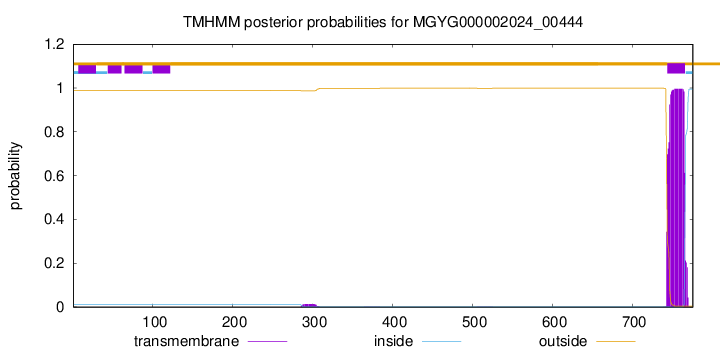
TMHMM annotations
Basic Information help
| Species | CAG-877 sp900554305 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; RF39; UBA660; CAG-877; CAG-877 sp900554305 | |||||||||||
| CAZyme ID | MGYG000002024_00444 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | D-inositol-3-phosphate glycosyltransferase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 40042; End: 42372 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 28 | 172 | 2e-28 | 0.8117647058823529 |
| GT4 | 469 | 614 | 6.5e-22 | 0.925 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd03801 | GT4_PimA-like | 5.84e-40 | 287 | 651 | 1 | 366 | phosphatidyl-myo-inositol mannosyltransferase. This family is most closely related to the GT4 family of glycosyltransferases and named after PimA in Propionibacterium freudenreichii, which is involved in the biosynthesis of phosphatidyl-myo-inositol mannosides (PIM) which are early precursors in the biosynthesis of lipomannans (LM) and lipoarabinomannans (LAM), and catalyzes the addition of a mannosyl residue from GDP-D-mannose (GDP-Man) to the position 2 of the carrier lipid phosphatidyl-myo-inositol (PI) to generate a phosphatidyl-myo-inositol bearing an alpha-1,2-linked mannose residue (PIM1). Glycosyltransferases catalyze the transfer of sugar moieties from activated donor molecules to specific acceptor molecules, forming glycosidic bonds. The acceptor molecule can be a lipid, a protein, a heterocyclic compound, or another carbohydrate residue. This group of glycosyltransferases is most closely related to the previously defined glycosyltransferase family 1 (GT1). The members of this family may transfer UDP, ADP, GDP, or CMP linked sugars. The diverse enzymatic activities among members of this family reflect a wide range of biological functions. The protein structure available for this family has the GTB topology, one of the two protein topologies observed for nucleotide-sugar-dependent glycosyltransferases. GTB proteins have distinct N- and C- terminal domains each containing a typical Rossmann fold. The two domains have high structural homology despite minimal sequence homology. The large cleft that separates the two domains includes the catalytic center and permits a high degree of flexibility. The members of this family are found mainly in certain bacteria and archaea. |
| cd00761 | Glyco_tranf_GTA_type | 4.52e-29 | 29 | 210 | 1 | 153 | Glycosyltransferase family A (GT-A) includes diverse families of glycosyl transferases with a common GT-A type structural fold. Glycosyltransferases (GTs) are enzymes that synthesize oligosaccharides, polysaccharides, and glycoconjugates by transferring the sugar moiety from an activated nucleotide-sugar donor to an acceptor molecule, which may be a growing oligosaccharide, a lipid, or a protein. Based on the stereochemistry of the donor and acceptor molecules, GTs are classified as either retaining or inverting enzymes. To date, all GT structures adopt one of two possible folds, termed GT-A fold and GT-B fold. This hierarchy includes diverse families of glycosyl transferases with a common GT-A type structural fold, which has two tightly associated beta/alpha/beta domains that tend to form a continuous central sheet of at least eight beta-strands. The majority of the proteins in this superfamily are Glycosyltransferase family 2 (GT-2) proteins. But it also includes families GT-43, GT-6, GT-8, GT13 and GT-7; which are evolutionarily related to GT-2 and share structure similarities. |
| COG0438 | RfaB | 5.11e-29 | 284 | 657 | 1 | 381 | Glycosyltransferase involved in cell wall bisynthesis [Cell wall/membrane/envelope biogenesis]. |
| cd03811 | GT4_GT28_WabH-like | 3.16e-27 | 287 | 593 | 1 | 309 | family 4 and family 28 glycosyltransferases similar to Klebsiella WabH. This family is most closely related to the GT1 family of glycosyltransferases. WabH in Klebsiella pneumoniae has been shown to transfer a GlcNAc residue from UDP-GlcNAc onto the acceptor GalUA residue in the cellular outer core. |
| pfam00535 | Glycos_transf_2 | 9.36e-25 | 28 | 160 | 1 | 131 | Glycosyl transferase family 2. Diverse family, transferring sugar from UDP-glucose, UDP-N-acetyl- galactosamine, GDP-mannose or CDP-abequose, to a range of substrates including cellulose, dolichol phosphate and teichoic acids. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QNR67047.1 | 1.95e-159 | 1 | 647 | 1 | 663 |
| AUO06976.1 | 4.73e-157 | 1 | 696 | 1 | 706 |
| AUO06971.1 | 9.31e-143 | 2 | 682 | 4 | 695 |
| QNR67052.1 | 2.60e-142 | 2 | 650 | 4 | 666 |
| AVM68009.1 | 6.06e-139 | 1 | 675 | 1 | 693 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5HEA_A | 2.05e-15 | 27 | 132 | 7 | 109 | CgTstructure in hexamer [Streptococcus parasanguinis FW213],5HEA_B CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEA_C CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEC_A CgT structure in dimer [Streptococcus parasanguinis FW213],5HEC_B CgT structure in dimer [Streptococcus parasanguinis FW213] |
| 6H1J_A | 7.03e-10 | 27 | 129 | 22 | 124 | ChainA, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H1J_B Chain B, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H1J_C Chain C, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H21_A Chain A, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H21_B Chain B, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H21_C Chain C, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H2N_A Chain A, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H2N_B Chain B, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H2N_C Chain C, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_A Chain A, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_B Chain B, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_C Chain C, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_D Chain D, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_E Chain E, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_F Chain F, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_G Chain G, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_H Chain H, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_I Chain I, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_O Chain O, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_P Chain P, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4F_Q Chain Q, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_A Chain A, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_B Chain B, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_C Chain C, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_D Chain D, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_E Chain E, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_F Chain F, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_G Chain G, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_H Chain H, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_I Chain I, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_O Chain O, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_P Chain P, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6H4M_Q Chain Q, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_A Chain A, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_B Chain B, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_C Chain C, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_D Chain D, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_E Chain E, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_F Chain F, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_G Chain G, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_H Chain H, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_I Chain I, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_O Chain O, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_P Chain P, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315],6HNQ_Q Chain Q, Probable ss-1,3-N-acetylglucosaminyltransferase [Staphylococcus aureus subsp. aureus N315] |
| 7MI0_A | 2.84e-09 | 277 | 655 | 9 | 394 | ChainA, Glycosyltransferase [Rickettsia africae ESF-5] |
| 5D00_A | 1.03e-07 | 440 | 593 | 168 | 321 | Crystalstructure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate and UMP [Bacillus subtilis subsp. subtilis str. 168],5D00_B Crystal structure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate and UMP [Bacillus subtilis subsp. subtilis str. 168],5D01_A Crystal structure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate [Bacillus subtilis subsp. subtilis str. 168],5D01_B Crystal structure of BshA from B. subtilis complexed with N-acetylglucosaminyl-malate [Bacillus subtilis subsp. subtilis str. 168] |
| 7EC2_A | 1.04e-07 | 473 | 627 | 316 | 465 | ChainA, Glycosyl transferase, group 1 family protein [Staphylococcus aureus subsp. aureus USA300],7EC2_B Chain B, Glycosyl transferase, group 1 family protein [Staphylococcus aureus subsp. aureus USA300] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O32268 | 5.42e-18 | 24 | 226 | 5 | 208 | Putative teichuronic acid biosynthesis glycosyltransferase TuaG OS=Bacillus subtilis (strain 168) OX=224308 GN=tuaG PE=2 SV=1 |
| P46915 | 8.38e-16 | 295 | 653 | 15 | 376 | Spore coat protein SA OS=Bacillus subtilis (strain 168) OX=224308 GN=cotSA PE=1 SV=1 |
| Q92IF9 | 2.54e-15 | 27 | 143 | 295 | 404 | Uncharacterized glycosyltransferase RC0461 OS=Rickettsia conorii (strain ATCC VR-613 / Malish 7) OX=272944 GN=RC0461 PE=3 SV=1 |
| Q4UM29 | 7.68e-15 | 27 | 143 | 295 | 404 | Uncharacterized glycosyltransferase RF_0543 OS=Rickettsia felis (strain ATCC VR-1525 / URRWXCal2) OX=315456 GN=RF_0543 PE=3 SV=1 |
| A0A0H2UR96 | 7.11e-14 | 27 | 182 | 5 | 158 | Glycosyltransferase GlyG OS=Streptococcus pneumoniae serotype 4 (strain ATCC BAA-334 / TIGR4) OX=170187 GN=glyG PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000049 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |