You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002478_01861
You are here: Home > Sequence: MGYG000002478_01861
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phocaeicola dorei | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Phocaeicola; Phocaeicola dorei | |||||||||||
| CAZyme ID | MGYG000002478_01861 | |||||||||||
| CAZy Family | GH137 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 2392905; End: 2394032 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH137 | 22 | 369 | 3.4e-169 | 0.9941176470588236 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd18610 | GH130_BT3780-like | 4.39e-08 | 59 | 191 | 80 | 191 | Glycosyl hydrolase family 130, such as beta-mammosidase BT3780 and BACOVA_03624. This subfamily contains glycosyl hydrolase family 130, as classified by the carbohydrate-active enzymes database (CAZY), and includes Bacteroides enzymes, BT3780 and BACOVA_03624. Members of this family possess 5-bladed beta-propeller domains similar to families 32, 43, 62, 68, 117 (GH32, GH43, GH62, GH68, GH117). GH130 enzymes are involved in the bacterial utilization of mannans or N-linked glycans. GH130 enzymes have also been shown to target beta-1,2- and beta-1,4-mannosidic linkages where these phosphorylases mediate bond cleavage by a single displacement reaction in which phosphate functions as the catalytic nucleophile. However, some lack the conserved basic residues that bind the phosphate nucleophile, as observed for the Bacteroides enzymes, BT3780 and BACOVA_03624, which are indeed beta-mannosidases that hydrolyze beta-1,2-mannosidic linkages through an inverting mechanism. |
| COG1621 | SacC | 1.37e-06 | 205 | 309 | 105 | 203 | Sucrose-6-phosphate hydrolase SacC, GH32 family [Carbohydrate transport and metabolism]. |
| cd18614 | GH130 | 7.20e-06 | 59 | 147 | 68 | 138 | Glycosyl hydrolase family 130; uncharacterized. This subfamily contains glycosyl hydrolase family 130 (GH130) proteins, as classified by the carbohydrate-active enzymes database (CAZY), most of which are as yet uncharacterized. GH130 enzymes are phosphorylases and hydrolases for beta-mannosides, and include beta-1,4-mannosylglucose phosphorylase (EC 2.4.1.281), beta-1,4-mannooligosaccharide phosphorylase (EC 2.4.1.319), beta-1,4-mannosyl-N-acetyl-glucosamine phosphorylase (EC 2.4.1.320), beta-1,2-mannobiose phosphorylase (EC 2.4.1.-), beta-1,2-oligomannan phosphorylase (EC 2.4.1.-) and beta-1,2-mannosidase (EC 3.2.1.-). They possess 5-bladed beta-propeller domains similar to families 32, 43, 62, 68, 117 (GH32, GH43, GH62, GH68, GH117). GH130 enzymes are involved in the bacterial utilization of mannans or N-linked glycans. Beta-1,4-mannosylglucose phosphorylase is involved in degradation of beta-1,4-D-mannosyl-N-acetyl-D-glucosamine linkages in the core of N-glycans; it produces alpha-mannose 1-phosphate and glucose from 4-O-beta-D-mannosyl-D-glucose and inorganic phosphate, using a critical catalytic Asp as a proton donor. |
| cd08994 | GH43_62_32_68_117_130-like | 1.35e-05 | 40 | 167 | 59 | 185 | Glycosyl hydrolase families: GH43, GH62, GH32, GH68, GH117, CH130. Members of the glycosyl hydrolase families 32, 43, 62, 68, 117 and 130 (GH32, GH43, GH62, GH68, GH117, GH130) all possess 5-bladed beta-propeller domains and comprise clans F and J, as classified by the carbohydrate-active enzymes database (CAZY). Clan F consists of families GH43 and GH62. GH43 includes beta-xylosidases (EC 3.2.1.37), beta-xylanases (EC 3.2.1.8), alpha-L-arabinases (EC 3.2.1.99), and alpha-L-arabinofuranosidases (EC 3.2.1.55), using aryl-glycosides as substrates, while family GH62 contains alpha-L-arabinofuranosidases (EC 3.2.1.55) that specifically cleave either alpha-1,2 or alpha-1,3-L-arabinofuranose sidechains from xylans. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Clan J consists of families GH32 and GH68. GH32 comprises sucrose-6-phosphate hydrolases, invertases (EC 3.2.1.26), inulinases (EC 3.2.1.7), levanases (EC 3.2.1.65), eukaryotic fructosyltransferases, and bacterial fructanotransferases while GH68 consists of frucosyltransferases (FTFs) that include levansucrase (EC 2.4.1.10); beta-fructofuranosidase (EC 3.2.1.26); inulosucrase (EC 2.4.1.9), while GH68 consists of frucosyltransferases (FTFs) that include levansucrase (EC 2.4.1.10); beta-fructofuranosidase (EC 3.2.1.26); inulosucrase (EC 2.4.1.9), all of which use sucrose as their preferential donor substrate. Members of this clan are retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) that catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. Structures of all families in the two clans manifest a funnel-shaped active site that comprises two subsites with a single route for access by ligands. Also included in this superfamily are GH117 enzymes that have exo-alpha-1,3-(3,6-anhydro)-l-galactosidase activity, removing terminal non-reducing alpha-1,3-linked 3,6-anhydro-l-galactose residues from their neoagarose substrate, and GH130 that are phosphorylases and hydrolases for beta-mannosides, involved in the bacterial utilization of mannans or N-linked glycans. |
| cd08994 | GH43_62_32_68_117_130-like | 5.78e-05 | 140 | 309 | 87 | 252 | Glycosyl hydrolase families: GH43, GH62, GH32, GH68, GH117, CH130. Members of the glycosyl hydrolase families 32, 43, 62, 68, 117 and 130 (GH32, GH43, GH62, GH68, GH117, GH130) all possess 5-bladed beta-propeller domains and comprise clans F and J, as classified by the carbohydrate-active enzymes database (CAZY). Clan F consists of families GH43 and GH62. GH43 includes beta-xylosidases (EC 3.2.1.37), beta-xylanases (EC 3.2.1.8), alpha-L-arabinases (EC 3.2.1.99), and alpha-L-arabinofuranosidases (EC 3.2.1.55), using aryl-glycosides as substrates, while family GH62 contains alpha-L-arabinofuranosidases (EC 3.2.1.55) that specifically cleave either alpha-1,2 or alpha-1,3-L-arabinofuranose sidechains from xylans. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Clan J consists of families GH32 and GH68. GH32 comprises sucrose-6-phosphate hydrolases, invertases (EC 3.2.1.26), inulinases (EC 3.2.1.7), levanases (EC 3.2.1.65), eukaryotic fructosyltransferases, and bacterial fructanotransferases while GH68 consists of frucosyltransferases (FTFs) that include levansucrase (EC 2.4.1.10); beta-fructofuranosidase (EC 3.2.1.26); inulosucrase (EC 2.4.1.9), while GH68 consists of frucosyltransferases (FTFs) that include levansucrase (EC 2.4.1.10); beta-fructofuranosidase (EC 3.2.1.26); inulosucrase (EC 2.4.1.9), all of which use sucrose as their preferential donor substrate. Members of this clan are retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) that catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. Structures of all families in the two clans manifest a funnel-shaped active site that comprises two subsites with a single route for access by ligands. Also included in this superfamily are GH117 enzymes that have exo-alpha-1,3-(3,6-anhydro)-l-galactosidase activity, removing terminal non-reducing alpha-1,3-linked 3,6-anhydro-l-galactose residues from their neoagarose substrate, and GH130 that are phosphorylases and hydrolases for beta-mannosides, involved in the bacterial utilization of mannans or N-linked glycans. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJR56806.1 | 1.21e-291 | 1 | 375 | 1 | 375 |
| QJR71225.1 | 1.21e-291 | 1 | 375 | 1 | 375 |
| ALA73595.1 | 1.21e-291 | 1 | 375 | 1 | 375 |
| QJR66885.1 | 1.21e-291 | 1 | 375 | 1 | 375 |
| QJR62627.1 | 1.21e-291 | 1 | 375 | 1 | 375 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5MUI_A | 3.11e-153 | 1 | 370 | 7 | 375 | Glycosidehydrolase BT_0996 [Bacteroides thetaiotaomicron VPI-5482] |
| 5MT2_A | 5.19e-147 | 17 | 370 | 22 | 375 | Glycosidehydrolase BT_0996 [Bacteroides thetaiotaomicron VPI-5482],5MUJ_A BT0996 RGII Chain B Complex [Bacteroides thetaiotaomicron VPI-5482] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
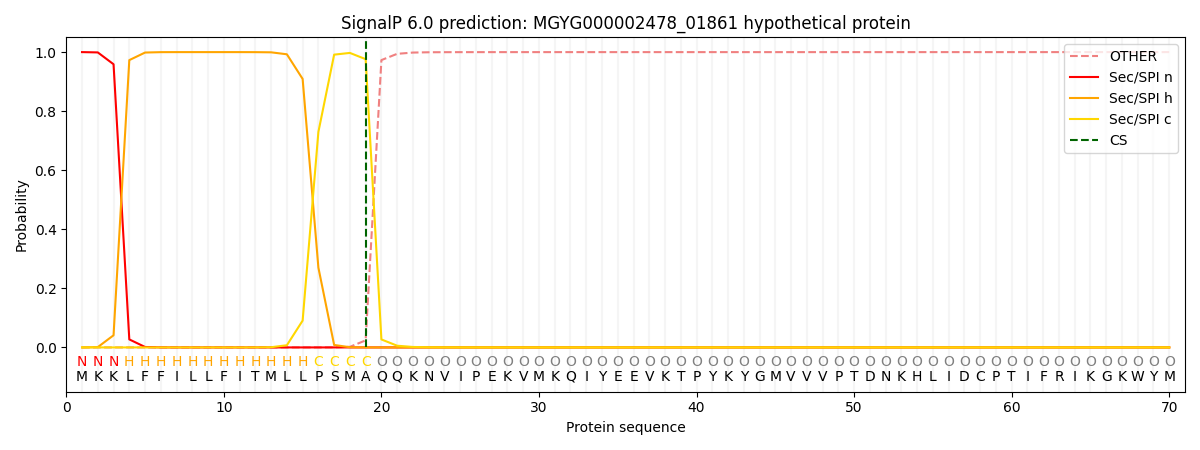
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000250 | 0.999075 | 0.000195 | 0.000152 | 0.000142 | 0.000135 |
