You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002548_01409
You are here: Home > Sequence: MGYG000002548_01409
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Staphylococcus argenteus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Staphylococcales; Staphylococcaceae; Staphylococcus; Staphylococcus argenteus | |||||||||||
| CAZyme ID | MGYG000002548_01409 | |||||||||||
| CAZy Family | CBM50 | |||||||||||
| CAZyme Description | N-acetylmuramoyl-L-alanine amidase sle1 | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 74575; End: 75372 Strand: - | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3942 | COG3942 | 4.91e-43 | 130 | 265 | 37 | 171 | Surface antigen [Cell wall/membrane/envelope biogenesis]. |
| PRK08581 | PRK08581 | 2.33e-23 | 161 | 264 | 508 | 616 | amidase domain-containing protein. |
| pfam05257 | CHAP | 3.74e-19 | 159 | 240 | 3 | 83 | CHAP domain. This domain corresponds to an amidase function. Many of these proteins are involved in cell wall metabolism of bacteria. This domain is found at the N-terminus of Escherichia coli gss, where it functions as a glutathionylspermidine amidase EC:3.5.1.78. This domain is found to be the catalytic domain of PlyCA. CHAP is the amidase domain of bifunctional Escherichia coli glutathionylspermidine synthetase/amidase, and it catalyzes the hydrolysis of Gsp (glutathionylspermidine) into glutathione and spermidine. |
| PRK06347 | PRK06347 | 8.97e-17 | 9 | 156 | 313 | 476 | 1,4-beta-N-acetylmuramoylhydrolase. |
| COG1388 | LysM | 2.91e-16 | 40 | 143 | 1 | 121 | LysM repeat [Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ATZ86539.1 | 3.99e-187 | 1 | 265 | 1 | 265 |
| BBN30373.1 | 3.99e-187 | 1 | 265 | 1 | 265 |
| ATY56297.1 | 3.99e-187 | 1 | 265 | 1 | 265 |
| CCE58466.1 | 3.99e-187 | 1 | 265 | 1 | 265 |
| BBD85475.1 | 3.99e-187 | 1 | 265 | 1 | 265 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2LRJ_A | 1.95e-38 | 153 | 265 | 2 | 112 | ChainA, Staphyloxanthin biosynthesis protein, putative [Staphylococcus aureus subsp. aureus COL] |
| 2K3A_A | 2.44e-38 | 159 | 265 | 50 | 153 | ChainA, CHAP domain protein [Staphylococcus saprophyticus subsp. saprophyticus ATCC 15305 = NCTC 7292] |
| 5T1Q_A | 4.09e-16 | 161 | 264 | 248 | 356 | ChainA, N-acetylmuramoyl-L-alanine amidase domain-containing protein SAOUHSC_02979 [Staphylococcus aureus subsp. aureus NCTC 8325],5T1Q_B Chain B, N-acetylmuramoyl-L-alanine amidase domain-containing protein SAOUHSC_02979 [Staphylococcus aureus subsp. aureus NCTC 8325],5T1Q_C Chain C, N-acetylmuramoyl-L-alanine amidase domain-containing protein SAOUHSC_02979 [Staphylococcus aureus subsp. aureus NCTC 8325],5T1Q_D Chain D, N-acetylmuramoyl-L-alanine amidase domain-containing protein SAOUHSC_02979 [Staphylococcus aureus subsp. aureus NCTC 8325] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q2G0D4 | 6.19e-176 | 1 | 265 | 1 | 265 | Probable autolysin SsaALP OS=Staphylococcus aureus (strain NCTC 8325 / PS 47) OX=93061 GN=SAOUHSC_00671 PE=1 SV=1 |
| Q5HRU2 | 1.77e-68 | 2 | 265 | 3 | 324 | N-acetylmuramoyl-L-alanine amidase sle1 OS=Staphylococcus epidermidis (strain ATCC 35984 / RP62A) OX=176279 GN=sle1 PE=3 SV=1 |
| Q8CMN2 | 1.77e-68 | 2 | 265 | 3 | 324 | N-acetylmuramoyl-L-alanine amidase sle1 OS=Staphylococcus epidermidis (strain ATCC 12228 / FDA PCI 1200) OX=176280 GN=sle1 PE=3 SV=1 |
| Q6GJK9 | 3.34e-68 | 29 | 265 | 93 | 334 | N-acetylmuramoyl-L-alanine amidase sle1 OS=Staphylococcus aureus (strain MRSA252) OX=282458 GN=sle1 PE=3 SV=1 |
| Q5HIL2 | 6.68e-68 | 29 | 265 | 93 | 334 | N-acetylmuramoyl-L-alanine amidase sle1 OS=Staphylococcus aureus (strain COL) OX=93062 GN=sle1 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
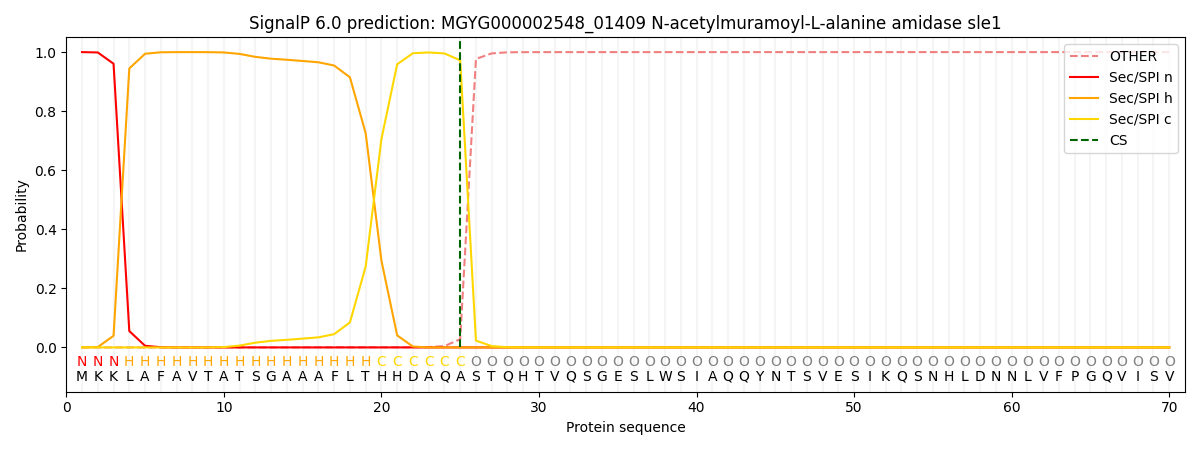
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000351 | 0.998848 | 0.000186 | 0.000221 | 0.000195 | 0.000164 |
