You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003137_01519
You are here: Home > Sequence: MGYG000003137_01519
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bradyrhizobium sp000015165 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Proteobacteria; Alphaproteobacteria; Rhizobiales; Xanthobacteraceae; Bradyrhizobium; Bradyrhizobium sp000015165 | |||||||||||
| CAZyme ID | MGYG000003137_01519 | |||||||||||
| CAZy Family | GT41 | |||||||||||
| CAZyme Description | Beta-barrel assembly-enhancing protease | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 231669; End: 233888 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT41 | 257 | 737 | 2e-108 | 0.5645390070921986 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3914 | Spy | 4.44e-74 | 33 | 735 | 6 | 618 | Predicted O-linked N-acetylglucosamine transferase, SPINDLY family [Posttranslational modification, protein turnover, chaperones]. |
| sd00006 | TPR | 6.60e-21 | 91 | 187 | 1 | 97 | Tetratricopeptide repeat. The Tetratricopeptide repeat (TPR) typically contains 34 amino acids and is found in a variety of organisms including bacteria, cyanobacteria, yeast, fungi, plants, and humans. It is present in a variety of proteins including those involved in chaperone, cell-cycle, transcription, and protein transport complexes. The number of TPR motifs varies among proteins. Those containing 5-6 tandem repeats generate a right-handed helical structure with an amphipathic channel that is thought to accommodate an alpha-helix of a target protein. It has been proposed that TPR proteins preferentially interact with WD-40 repeat proteins, but in many instances several TPR-proteins seem to aggregate to multi-protein complexes. |
| sd00006 | TPR | 1.68e-20 | 58 | 153 | 2 | 97 | Tetratricopeptide repeat. The Tetratricopeptide repeat (TPR) typically contains 34 amino acids and is found in a variety of organisms including bacteria, cyanobacteria, yeast, fungi, plants, and humans. It is present in a variety of proteins including those involved in chaperone, cell-cycle, transcription, and protein transport complexes. The number of TPR motifs varies among proteins. Those containing 5-6 tandem repeats generate a right-handed helical structure with an amphipathic channel that is thought to accommodate an alpha-helix of a target protein. It has been proposed that TPR proteins preferentially interact with WD-40 repeat proteins, but in many instances several TPR-proteins seem to aggregate to multi-protein complexes. |
| pfam13844 | Glyco_transf_41 | 3.39e-20 | 364 | 721 | 61 | 540 | Glycosyl transferase family 41. This family of glycosyltransferases includes O-linked beta-N-acetylglucosamine (O-GlcNAc) transferase, an enzyme which catalyzes the addition of O-GlcNAc to serine and threonine residues. In addition to its function as an O-GlcNAc transferase, human OGT also appears to proteolytically cleave the epigenetic cell-cycle regulator HCF-1. |
| TIGR02917 | PEP_TPR_lipo | 1.93e-19 | 21 | 335 | 20 | 403 | putative PEP-CTERM system TPR-repeat lipoprotein. This protein family occurs in strictly within a subset of Gram-negative bacterial species with the proposed PEP-CTERM/exosortase system, analogous to the LPXTG/sortase system common in Gram-positive bacteria. This protein occurs in a species if and only if a transmembrane histidine kinase (TIGR02916) and a DNA-binding response regulator (TIGR02915) also occur. The present of tetratricopeptide repeats (TPR) suggests protein-protein interaction, possibly for the regulation of PEP-CTERM protein expression, since many PEP-CTERM proteins in these genomes are preceded by a proposed DNA binding site for the response regulator. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ABQ37575.1 | 0.0 | 1 | 739 | 1 | 739 |
| BAM88544.1 | 2.57e-287 | 1 | 738 | 1 | 738 |
| SMX57226.1 | 6.84e-285 | 1 | 738 | 1 | 738 |
| CAL78844.1 | 1.48e-271 | 1 | 738 | 1 | 738 |
| ABQ37577.1 | 8.74e-253 | 1 | 739 | 1 | 739 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5DJS_A | 1.74e-74 | 301 | 738 | 83 | 522 | Thermobaculumterrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum],5DJS_B Thermobaculum terrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum],5DJS_C Thermobaculum terrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum],5DJS_D Thermobaculum terrenum O-GlcNAc transferase mutant - K341M [Thermobaculum terrenum] |
| 2VSN_A | 1.34e-23 | 369 | 720 | 197 | 548 | Structureand topological arrangement of an O-GlcNAc transferase homolog: insight into molecular control of intracellular glycosylation [Xanthomonas campestris pv. campestris str. 8004],2VSN_B Structure and topological arrangement of an O-GlcNAc transferase homolog: insight into molecular control of intracellular glycosylation [Xanthomonas campestris pv. campestris str. 8004] |
| 2JLB_A | 2.36e-23 | 369 | 720 | 197 | 548 | Xanthomonascampestris putative OGT (XCC0866), complex with UDP- GlcNAc phosphonate analogue [Xanthomonas campestris pv. campestris],2JLB_B Xanthomonas campestris putative OGT (XCC0866), complex with UDP- GlcNAc phosphonate analogue [Xanthomonas campestris pv. campestris],2VSY_A Xanthomonas campestris putative OGT (XCC0866), apostructure [Xanthomonas campestris pv. campestris str. ATCC 33913],2VSY_B Xanthomonas campestris putative OGT (XCC0866), apostructure [Xanthomonas campestris pv. campestris str. ATCC 33913],2XGM_A Substrate and product analogues as human O-GlcNAc transferase inhibitors. [Xanthomonas campestris],2XGM_B Substrate and product analogues as human O-GlcNAc transferase inhibitors. [Xanthomonas campestris],2XGO_A XcOGT in complex with UDP-S-GlcNAc [Xanthomonas campestris],2XGO_B XcOGT in complex with UDP-S-GlcNAc [Xanthomonas campestris],2XGS_A XcOGT in complex with C-UDP [Xanthomonas campestris],2XGS_B XcOGT in complex with C-UDP [Xanthomonas campestris] |
| 6Q4M_A | 1.37e-20 | 365 | 738 | 219 | 712 | Crystalstructure of the O-GlcNAc transferase Asn648Tyr mutation [Homo sapiens] |
| 5NPS_A | 2.37e-20 | 365 | 738 | 214 | 707 | Thehuman O-GlcNAc transferase in complex with a bisubstrate inhibitor [Homo sapiens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O82039 | 3.29e-59 | 322 | 736 | 429 | 850 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Petunia hybrida OX=4102 GN=SPY PE=2 SV=1 |
| Q8RVB2 | 1.51e-58 | 322 | 736 | 429 | 850 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Solanum lycopersicum OX=4081 GN=SPY PE=2 SV=1 |
| Q6YZI0 | 2.70e-58 | 315 | 736 | 408 | 836 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Oryza sativa subsp. japonica OX=39947 GN=SPY PE=2 SV=1 |
| Q96301 | 2.08e-57 | 323 | 736 | 425 | 845 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Arabidopsis thaliana OX=3702 GN=SPY PE=1 SV=1 |
| O82422 | 5.01e-54 | 323 | 736 | 416 | 836 | Probable UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase SPINDLY OS=Hordeum vulgare OX=4513 GN=SPY PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
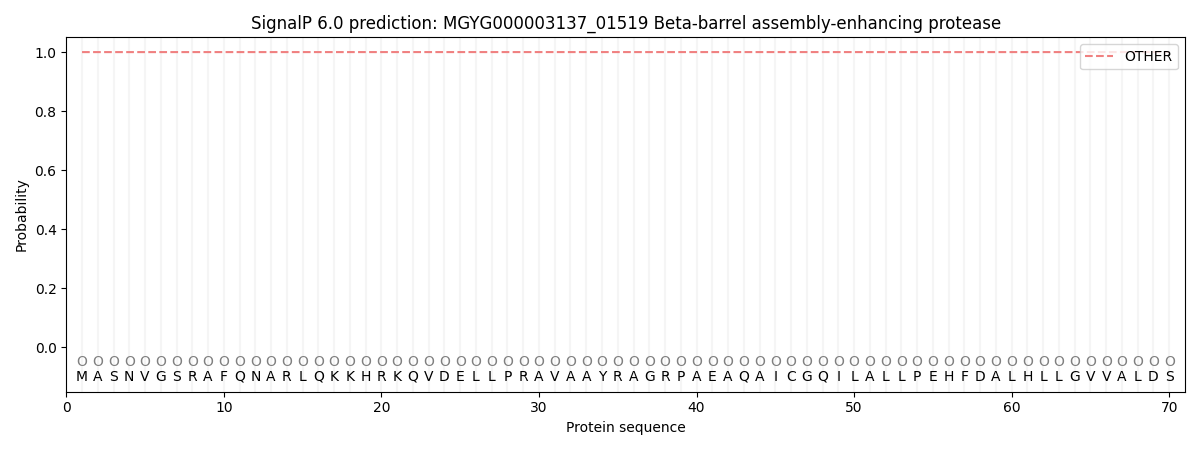
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000046 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |
