You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003317_01248
You are here: Home > Sequence: MGYG000003317_01248
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
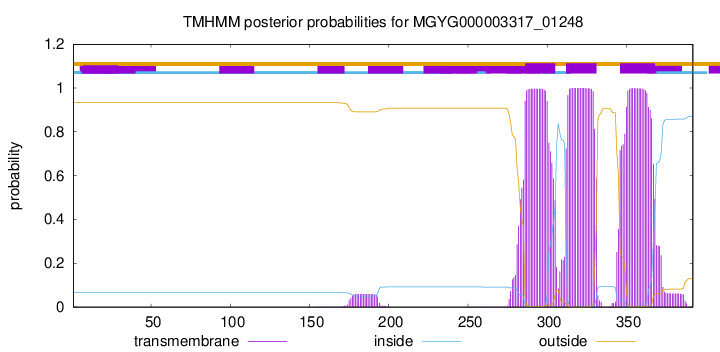
TMHMM annotations
Basic Information help
| Species | Lentilactobacillus buchneri | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae; Lentilactobacillus; Lentilactobacillus buchneri | |||||||||||
| CAZyme ID | MGYG000003317_01248 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | Poly-beta-1,6-N-acetyl-D-glucosamine synthase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 22092; End: 23270 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 29 | 255 | 7.7e-33 | 0.9826086956521739 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| PRK11204 | PRK11204 | 1.12e-93 | 12 | 307 | 37 | 331 | N-glycosyltransferase; Provisional |
| PRK14583 | hmsR | 1.99e-68 | 4 | 305 | 49 | 350 | poly-beta-1,6 N-acetyl-D-glucosamine synthase. |
| COG1215 | BcsA | 3.94e-58 | 29 | 304 | 56 | 332 | Glycosyltransferase, catalytic subunit of cellulose synthase and poly-beta-1,6-N-acetylglucosamine synthase [Cell motility]. |
| cd06423 | CESA_like | 6.20e-55 | 31 | 212 | 1 | 180 | CESA_like is the cellulose synthase superfamily. The cellulose synthase (CESA) superfamily includes a wide variety of glycosyltransferase family 2 enzymes that share the common characteristic of catalyzing the elongation of polysaccharide chains. The members include cellulose synthase catalytic subunit, chitin synthase, glucan biosynthesis protein and other families of CESA-like proteins. Cellulose synthase catalyzes the polymerization reaction of cellulose, an aggregate of unbranched polymers of beta-1,4-linked glucose residues in plants, most algae, some bacteria and fungi, and even some animals. In bacteria, algae and lower eukaryotes, there is a second unrelated type of cellulose synthase (Type II), which produces acylated cellulose, a derivative of cellulose. Chitin synthase catalyzes the incorporation of GlcNAc from substrate UDP-GlcNAc into chitin, which is a linear homopolymer of beta-(1,4)-linked GlcNAc residues and Glucan Biosynthesis protein catalyzes the elongation of beta-1,2 polyglucose chains of Glucan. |
| pfam00535 | Glycos_transf_2 | 2.10e-33 | 30 | 198 | 1 | 164 | Glycosyl transferase family 2. Diverse family, transferring sugar from UDP-glucose, UDP-N-acetyl- galactosamine, GDP-mannose or CDP-abequose, to a range of substrates including cellulose, dolichol phosphate and teichoic acids. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AFR99312.1 | 3.10e-260 | 4 | 367 | 22 | 385 |
| AEB72446.1 | 4.40e-260 | 4 | 367 | 22 | 385 |
| QOP50411.1 | 1.40e-233 | 4 | 367 | 22 | 385 |
| QOJ85745.1 | 1.40e-233 | 4 | 367 | 22 | 385 |
| APR07015.1 | 1.40e-233 | 4 | 367 | 22 | 385 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2Z87_A | 5.43e-10 | 28 | 144 | 375 | 487 | Crystalstructure of chondroitin polymerase from Escherichia coli strain K4 (K4CP) complexed with UDP-GalNAc and UDP [Escherichia coli],2Z87_B Crystal structure of chondroitin polymerase from Escherichia coli strain K4 (K4CP) complexed with UDP-GalNAc and UDP [Escherichia coli] |
| 2Z86_A | 5.44e-10 | 28 | 144 | 376 | 488 | Crystalstructure of chondroitin polymerase from Escherichia coli strain K4 (K4CP) complexed with UDP-GlcUA and UDP [Escherichia coli],2Z86_B Crystal structure of chondroitin polymerase from Escherichia coli strain K4 (K4CP) complexed with UDP-GlcUA and UDP [Escherichia coli],2Z86_C Crystal structure of chondroitin polymerase from Escherichia coli strain K4 (K4CP) complexed with UDP-GlcUA and UDP [Escherichia coli],2Z86_D Crystal structure of chondroitin polymerase from Escherichia coli strain K4 (K4CP) complexed with UDP-GlcUA and UDP [Escherichia coli] |
| 5HEA_A | 5.45e-10 | 27 | 138 | 5 | 112 | CgTstructure in hexamer [Streptococcus parasanguinis FW213],5HEA_B CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEA_C CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEC_A CgT structure in dimer [Streptococcus parasanguinis FW213],5HEC_B CgT structure in dimer [Streptococcus parasanguinis FW213] |
| 3BCV_A | 1.06e-09 | 29 | 126 | 7 | 100 | Crystalstructure of a putative glycosyltransferase from Bacteroides fragilis [Bacteroides fragilis NCTC 9343],3BCV_B Crystal structure of a putative glycosyltransferase from Bacteroides fragilis [Bacteroides fragilis NCTC 9343] |
| 5EKE_A | 1.28e-09 | 29 | 120 | 28 | 119 | Structureof the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKE_B Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKE_C Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa],5EKE_D Structure of the polyisoprenyl-phosphate glycosyltransferase GtrB (F215A mutant) [Synechocystis sp. PCC 6803 substr. Kazusa] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q5HKQ0 | 3.57e-68 | 5 | 371 | 23 | 402 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus epidermidis (strain ATCC 35984 / RP62A) OX=176279 GN=icaA PE=1 SV=1 |
| Q8GLC5 | 3.57e-68 | 5 | 371 | 23 | 402 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus epidermidis OX=1282 GN=icaA PE=3 SV=1 |
| Q6G608 | 1.09e-66 | 5 | 299 | 23 | 311 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus aureus (strain MSSA476) OX=282459 GN=icaA PE=3 SV=1 |
| Q8NUI7 | 1.09e-66 | 5 | 299 | 23 | 311 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus aureus (strain MW2) OX=196620 GN=icaA PE=3 SV=1 |
| Q5HCN1 | 4.27e-66 | 5 | 299 | 23 | 311 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus aureus (strain COL) OX=93062 GN=icaA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
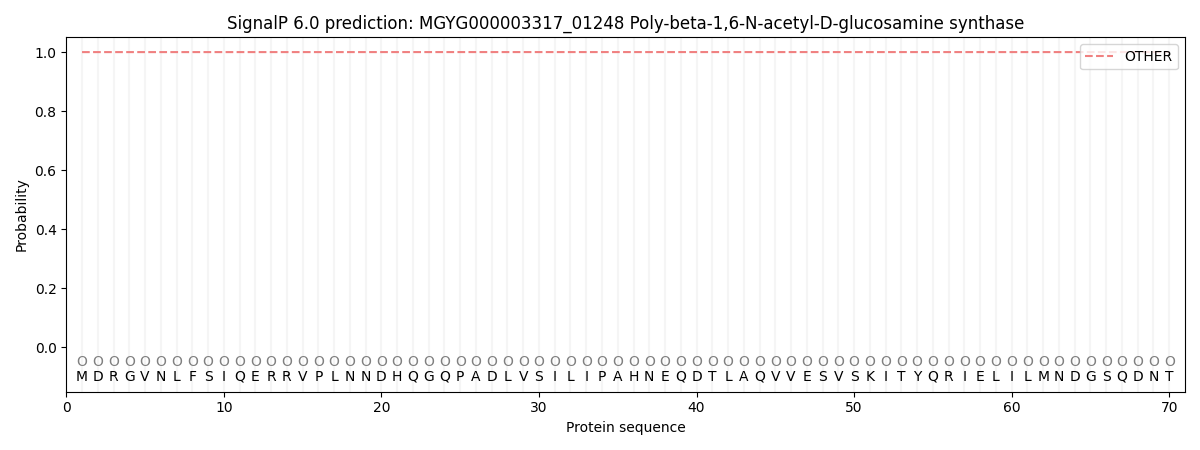
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000047 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |