You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003351_01025
You are here: Home > Sequence: MGYG000003351_01025
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides sp900765785 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides sp900765785 | |||||||||||
| CAZyme ID | MGYG000003351_01025 | |||||||||||
| CAZy Family | GH36 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 45257; End: 48352 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH36 | 26 | 699 | 1e-201 | 0.9956395348837209 |
| GH43 | 742 | 998 | 7.2e-104 | 0.9878048780487805 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd18825 | GH43_CtGH43-like | 5.81e-159 | 734 | 1014 | 1 | 285 | Glycosyl hydrolase family 43 protein similar to Clostridium thermocellum exo-beta-1,3-galactanase CtGH43 and Ruminococcus champanellensis arabinanase Ara43A. This uncharacterized glycosyl hydrolase family 43 (GH43) subgroup belongs to a subgroup which includes characterized enzymes with exo-beta-1,3-galactanase (EC 3.2.1.145, also known as galactan 1,3-beta-galactosidase) activity such as Clostridium thermocellum (Ct1,3Gal43A or CtGH43) and Phanerochaete chrysosporium 1,3Gal43A (Pc1, 3Gal43A), and arabinanase (EC 3.2.1.99) activity such as Ruminococcus champanellensis Ara43A. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| cd08985 | GH43_CtGH43-like | 1.46e-117 | 734 | 1014 | 1 | 273 | Glycosyl hydrolase family 43 protein such as Clostridium thermocellum exo-beta-1,3-galactanase CtGH43 and Ruminococcus champanellensis arabinanase Ara43A. This glycosyl hydrolase family 43 (GH43) subgroup includes characterized enzymes with exo-beta-1,3-galactanase (EC 3.2.1.145, also known as galactan 1,3-beta-galactosidase) activity such as Clostridium thermocellum (Ct1,3Gal43A or CtGH43) and Phanerochaete chrysosporium 1,3Gal43A (Pc1, 3Gal43A), and arabinanase (EC 3.2.1.99) activity such as Ruminococcus champanellensis Ara43A. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| pfam02065 | Melibiase | 3.59e-113 | 271 | 612 | 1 | 347 | Melibiase. Glycoside hydrolase families GH27, GH31 and GH36 form the glycoside hydrolase clan GH-D. Glycoside hydrolase family 36 can be split into 11 families, GH36A to GH36K. This family includes enzymes from GH36A-B and GH36D-K and from GH27. |
| COG3345 | GalA | 3.26e-110 | 26 | 715 | 4 | 682 | Alpha-galactosidase [Carbohydrate transport and metabolism]. |
| cd18826 | GH43_CtGH43-like | 1.70e-109 | 734 | 1014 | 1 | 269 | Glycosyl hydrolase family 43 protein similar to Clostridium thermocellum exo-beta-1,3-galactanase CtGH43 and Ruminococcus champanellensis arabinanase Ara43A. This uncharacterized glycosyl hydrolase family 43 (GH43) subgroup belongs to a subgroup which includes characterized enzymes with exo-beta-1,3-galactanase (EC 3.2.1.145, also known as galactan 1,3-beta-galactosidase) activity such as Clostridium thermocellum (Ct1,3Gal43A or CtGH43) and Phanerochaete chrysosporium 1,3Gal43A (Pc1, 3Gal43A), and arabinanase (EC 3.2.1.99) activity such as Ruminococcus champanellensis Ara43A. GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT48223.1 | 0.0 | 1 | 717 | 1 | 719 |
| QNL40042.1 | 0.0 | 1 | 716 | 1 | 718 |
| AII65838.1 | 0.0 | 1 | 716 | 1 | 718 |
| QGT72605.1 | 0.0 | 1 | 716 | 11 | 728 |
| ALA72663.1 | 0.0 | 1 | 716 | 1 | 718 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4FNQ_A | 2.84e-108 | 70 | 698 | 76 | 708 | Crystalstructure of GH36 alpha-galactosidase AgaB from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
| 4FNR_A | 2.84e-108 | 70 | 698 | 76 | 708 | Crystalstructure of GH36 alpha-galactosidase AgaA from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4FNR_B Crystal structure of GH36 alpha-galactosidase AgaA from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4FNR_C Crystal structure of GH36 alpha-galactosidase AgaA from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4FNR_D Crystal structure of GH36 alpha-galactosidase AgaA from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
| 4FNP_A | 2.09e-106 | 70 | 698 | 76 | 708 | Crystalstructure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4FNP_B Crystal structure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4FNP_C Crystal structure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4FNP_D Crystal structure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4FNS_A Crystal structure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus in complex with 1-deoxygalactonojirimycin [Geobacillus stearothermophilus],4FNS_B Crystal structure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus in complex with 1-deoxygalactonojirimycin [Geobacillus stearothermophilus],4FNS_C Crystal structure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus in complex with 1-deoxygalactonojirimycin [Geobacillus stearothermophilus],4FNS_D Crystal structure of GH36 alpha-galactosidase AgaA A355E from Geobacillus stearothermophilus in complex with 1-deoxygalactonojirimycin [Geobacillus stearothermophilus] |
| 4FNU_A | 2.09e-106 | 70 | 698 | 76 | 708 | Crystalstructure of GH36 alpha-galactosidase AgaA A355E D478A from Geobacillus stearothermophilus in complex with stachyose [Geobacillus stearothermophilus],4FNU_B Crystal structure of GH36 alpha-galactosidase AgaA A355E D478A from Geobacillus stearothermophilus in complex with stachyose [Geobacillus stearothermophilus],4FNU_C Crystal structure of GH36 alpha-galactosidase AgaA A355E D478A from Geobacillus stearothermophilus in complex with stachyose [Geobacillus stearothermophilus],4FNU_D Crystal structure of GH36 alpha-galactosidase AgaA A355E D478A from Geobacillus stearothermophilus in complex with stachyose [Geobacillus stearothermophilus] |
| 4FNT_A | 2.92e-106 | 70 | 698 | 76 | 708 | Crystalstructure of GH36 alpha-galactosidase AgaA A355E D548N from Geobacillus stearothermophilus in complex with raffinose [Geobacillus stearothermophilus],4FNT_B Crystal structure of GH36 alpha-galactosidase AgaA A355E D548N from Geobacillus stearothermophilus in complex with raffinose [Geobacillus stearothermophilus],4FNT_C Crystal structure of GH36 alpha-galactosidase AgaA A355E D548N from Geobacillus stearothermophilus in complex with raffinose [Geobacillus stearothermophilus],4FNT_D Crystal structure of GH36 alpha-galactosidase AgaA A355E D548N from Geobacillus stearothermophilus in complex with raffinose [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P43467 | 2.36e-108 | 70 | 707 | 78 | 722 | Alpha-galactosidase 1 OS=Pediococcus pentosaceus OX=1255 GN=agaR PE=3 SV=1 |
| Q9ALJ4 | 1.56e-107 | 70 | 698 | 76 | 708 | Alpha-galactosidase AgaA OS=Geobacillus stearothermophilus OX=1422 GN=agaA PE=1 SV=1 |
| Q0CVH2 | 6.60e-107 | 14 | 698 | 23 | 729 | Probable alpha-galactosidase C OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=aglC PE=3 SV=1 |
| Q5AU92 | 1.91e-106 | 74 | 710 | 103 | 744 | Alpha-galactosidase C OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=aglC PE=1 SV=1 |
| P27756 | 1.27e-105 | 28 | 700 | 21 | 703 | Alpha-galactosidase OS=Streptococcus mutans serotype c (strain ATCC 700610 / UA159) OX=210007 GN=aga PE=3 SV=3 |
SignalP and Lipop Annotations help
This protein is predicted as SP
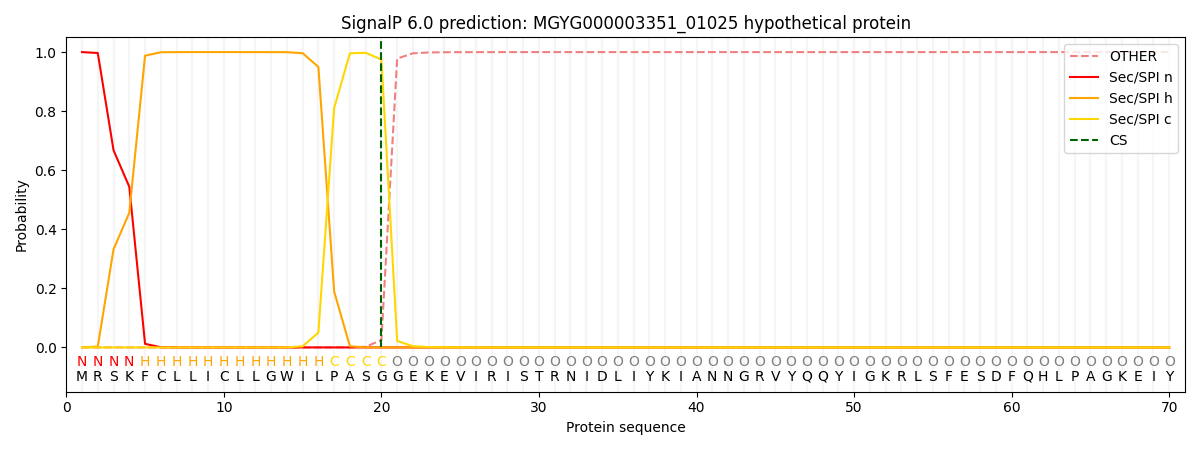
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000229 | 0.999183 | 0.000183 | 0.000138 | 0.000139 | 0.000130 |
