You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003423_00805
You are here: Home > Sequence: MGYG000003423_00805
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
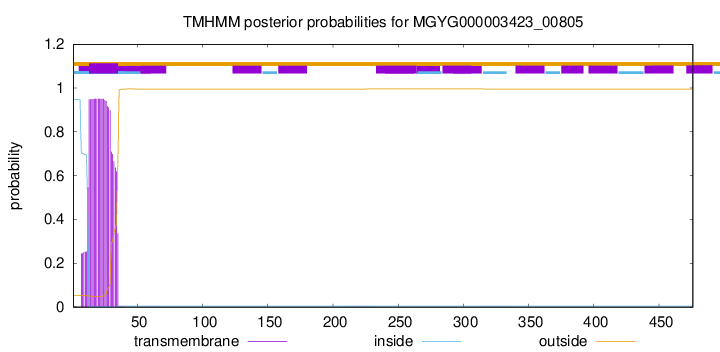
TMHMM annotations
Basic Information help
| Species | W3P20-009 sp900766825 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; W3P20-009; W3P20-009; W3P20-009 sp900766825 | |||||||||||
| CAZyme ID | MGYG000003423_00805 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 50637; End: 52067 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 163 | 352 | 3.9e-40 | 0.8861386138613861 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| smart00656 | Amb_all | 3.37e-29 | 194 | 350 | 28 | 186 | Amb_all domain. |
| COG3866 | PelB | 2.47e-24 | 29 | 410 | 14 | 342 | Pectate lyase [Carbohydrate transport and metabolism]. |
| pfam00544 | Pec_lyase_C | 8.86e-12 | 198 | 350 | 52 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AAW84045.1 | 9.44e-167 | 86 | 476 | 1 | 389 |
| ADE83376.1 | 9.32e-119 | 15 | 411 | 14 | 421 |
| QVJ81552.1 | 1.87e-118 | 15 | 411 | 14 | 421 |
| AZA53390.1 | 1.38e-45 | 142 | 411 | 83 | 362 |
| QXU49699.1 | 1.38e-45 | 10 | 411 | 1 | 362 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1VBL_A | 4.72e-16 | 186 | 377 | 136 | 340 | Structureof the thermostable pectate lyase PL 47 [Bacillus sp. TS-47] |
| 3ZSC_A | 2.19e-14 | 202 | 327 | 87 | 214 | Catalyticfunction and substrate recognition of the pectate lyase from Thermotoga maritima [Thermotoga maritima] |
| 1PXZ_A | 4.46e-09 | 201 | 344 | 127 | 262 | ChainA, Major pollen allergen Jun a 1 [Juniperus ashei],1PXZ_B Chain B, Major pollen allergen Jun a 1 [Juniperus ashei] |
| 1PCL_A | 4.45e-07 | 158 | 408 | 59 | 351 | ChainA, PECTATE LYASE E [Dickeya chrysanthemi] |
| 4HWV_A | 5.65e-06 | 207 | 337 | 194 | 364 | Structureof Pectate Lyase from Acidovorax avenae subsp citrulli [Acidovorax citrulli AAC00-1],4HWV_B Structure of Pectate Lyase from Acidovorax avenae subsp citrulli [Acidovorax citrulli AAC00-1] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| B1L969 | 4.88e-18 | 202 | 327 | 112 | 239 | Pectate trisaccharide-lyase OS=Thermotoga sp. (strain RQ2) OX=126740 GN=pelA PE=3 SV=1 |
| Q9WYR4 | 1.21e-17 | 202 | 327 | 114 | 241 | Pectate trisaccharide-lyase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=pelA PE=1 SV=1 |
| Q9C5M8 | 2.45e-10 | 227 | 354 | 208 | 336 | Probable pectate lyase 18 OS=Arabidopsis thaliana OX=3702 GN=At4g24780 PE=2 SV=2 |
| Q93WF1 | 2.53e-10 | 227 | 354 | 217 | 345 | Probable pectate lyase 20 OS=Arabidopsis thaliana OX=3702 GN=At5g48900 PE=2 SV=1 |
| Q9M8Z8 | 1.39e-09 | 227 | 354 | 216 | 344 | Probable pectate lyase 8 OS=Arabidopsis thaliana OX=3702 GN=At3g07010 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
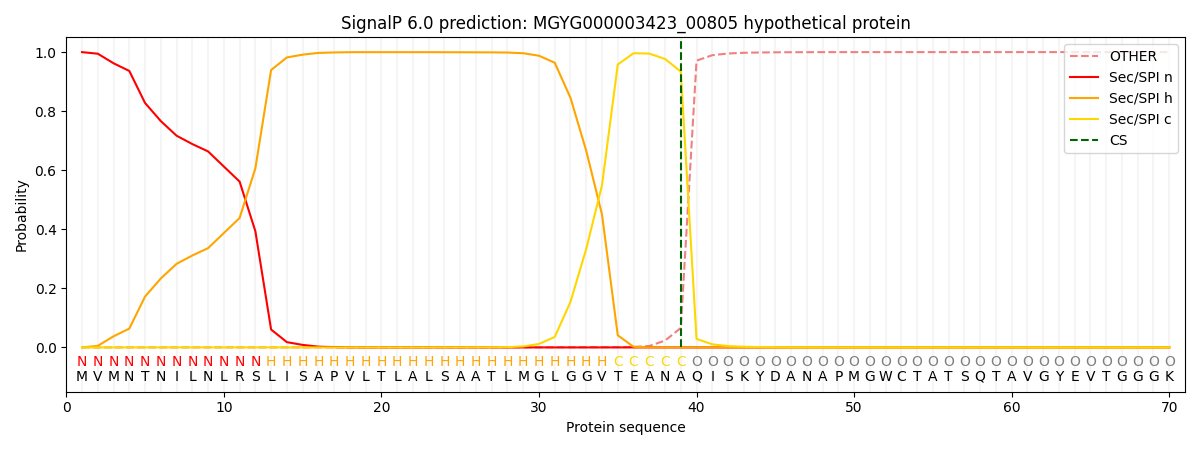
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000500 | 0.998605 | 0.000209 | 0.000279 | 0.000188 | 0.000178 |