You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003525_00794
You are here: Home > Sequence: MGYG000003525_00794
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | RC9 sp004556005 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; UBA932; RC9; RC9 sp004556005 | |||||||||||
| CAZyme ID | MGYG000003525_00794 | |||||||||||
| CAZy Family | GH29 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 162310; End: 163740 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH29 | 8 | 369 | 7.5e-93 | 0.9682080924855492 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam01120 | Alpha_L_fucos | 2.13e-94 | 27 | 365 | 6 | 333 | Alpha-L-fucosidase. |
| smart00812 | Alpha_L_fucos | 4.76e-85 | 27 | 397 | 7 | 372 | Alpha-L-fucosidase. O-Glycosyl hydrolases (EC 3.2.1.-) are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycosyl hydrolases, based on sequence similarity, has led to the definition of 85 different families. This classification is available on the CAZy (CArbohydrate-Active EnZymes) web site. Because the fold of proteins is better conserved than their sequences, some of the families can be grouped in 'clans'. Family 29 encompasses alpha-L-fucosidases, which is a lysosomal enzyme responsible for hydrolyzing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins. Deficiency of alpha-L-fucosidase results in the lysosomal storage disease fucosidosis. |
| COG3669 | AfuC | 4.57e-38 | 52 | 393 | 1 | 349 | Alpha-L-fucosidase [Carbohydrate transport and metabolism]. |
| pfam16757 | Fucosidase_C | 0.001 | 380 | 473 | 7 | 89 | Alpha-L-fucosidase C-terminal domain. The C-terminal domain of Structure 1hl8 is constructed of eight anti-parallel-strands packed into two-sheets of five and three strands, respectively, forming a two-layer-sandwich containing a Greek key motif. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJB41770.1 | 5.95e-155 | 15 | 474 | 17 | 474 |
| QJB35235.1 | 8.43e-155 | 15 | 474 | 17 | 474 |
| QUT88497.1 | 8.82e-155 | 27 | 464 | 37 | 458 |
| ALJ60491.1 | 8.82e-155 | 27 | 464 | 37 | 458 |
| QEC61633.1 | 5.51e-152 | 6 | 473 | 4 | 473 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6GN6_A | 1.13e-63 | 38 | 396 | 30 | 379 | Alpha-L-fucosidaseisoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_B Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_C Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_D Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_E Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus],6GN6_F Alpha-L-fucosidase isoenzyme 1 from Paenibacillus thiaminolyticus [Paenibacillus thiaminolyticus] |
| 4JL2_A | 4.30e-38 | 39 | 392 | 19 | 372 | Crystalstructure of a bacterial fucosidase with a monovalent iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482],4JL2_B Crystal structure of a bacterial fucosidase with a monovalent iminocyclitol inhibitor [Bacteroides thetaiotaomicron VPI-5482] |
| 4PCS_A | 4.94e-38 | 39 | 392 | 19 | 372 | Crystalstructure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCS_B Crystal structure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCS_C Crystal structure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCS_D Crystal structure of a bacterial fucosidase with iminosugar (2S,3S,4R,5S)-3,4-dihydroxy-2-[2'-phenyl]ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_A Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_B Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_C Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482],4PCT_D Crystal structure of a bacterial fucosidase with iminocyclitol (2S,3S,4R,5S)-3,4-dihydroxy-2-ethynyl-5-methylpyrrolidine [Bacteroides thetaiotaomicron VPI-5482] |
| 2WVT_A | 5.28e-38 | 39 | 392 | 23 | 376 | Crystalstructure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron in complex with a novel iminosugar fucosidase inhibitor [Bacteroides thetaiotaomicron VPI-5482],2WVT_B Crystal structure of an alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron in complex with a novel iminosugar fucosidase inhibitor [Bacteroides thetaiotaomicron VPI-5482],2WVU_A Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482],2WVU_B Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482],2WVU_C Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482],2WVU_D Crystal structure of a Michaelis complex of alpha-L-fucosidase GH29 from Bacteroides thetaiotaomicron with the synthetic substrate 4- nitrophenyl-alpha-L-fucose [Bacteroides thetaiotaomicron VPI-5482] |
| 2WVS_A | 5.28e-38 | 39 | 392 | 23 | 376 | Crystalstructure of an alpha-L-fucosidase GH29 trapped covalent intermediate from Bacteroides thetaiotaomicron in complex with 2- fluoro-fucosyl fluoride using an E288Q mutant [Bacteroides thetaiotaomicron VPI-5482],2WVS_B Crystal structure of an alpha-L-fucosidase GH29 trapped covalent intermediate from Bacteroides thetaiotaomicron in complex with 2- fluoro-fucosyl fluoride using an E288Q mutant [Bacteroides thetaiotaomicron VPI-5482],2WVS_C Crystal structure of an alpha-L-fucosidase GH29 trapped covalent intermediate from Bacteroides thetaiotaomicron in complex with 2- fluoro-fucosyl fluoride using an E288Q mutant [Bacteroides thetaiotaomicron VPI-5482],2WVS_D Crystal structure of an alpha-L-fucosidase GH29 trapped covalent intermediate from Bacteroides thetaiotaomicron in complex with 2- fluoro-fucosyl fluoride using an E288Q mutant [Bacteroides thetaiotaomicron VPI-5482] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P04066 | 2.20e-37 | 7 | 473 | 15 | 462 | Tissue alpha-L-fucosidase OS=Homo sapiens OX=9606 GN=FUCA1 PE=1 SV=4 |
| Q99LJ1 | 3.35e-37 | 19 | 475 | 17 | 450 | Tissue alpha-L-fucosidase OS=Mus musculus OX=10090 GN=Fuca1 PE=1 SV=1 |
| C3YWU0 | 1.15e-36 | 10 | 468 | 3 | 440 | Alpha-L-fucosidase OS=Branchiostoma floridae OX=7739 GN=BRAFLDRAFT_56888 PE=3 SV=2 |
| Q60HF8 | 2.93e-36 | 5 | 473 | 15 | 464 | Tissue alpha-L-fucosidase OS=Macaca fascicularis OX=9541 GN=FUCA1 PE=2 SV=1 |
| Q2KIM0 | 9.67e-35 | 42 | 473 | 56 | 464 | Tissue alpha-L-fucosidase OS=Bos taurus OX=9913 GN=FUCA1 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
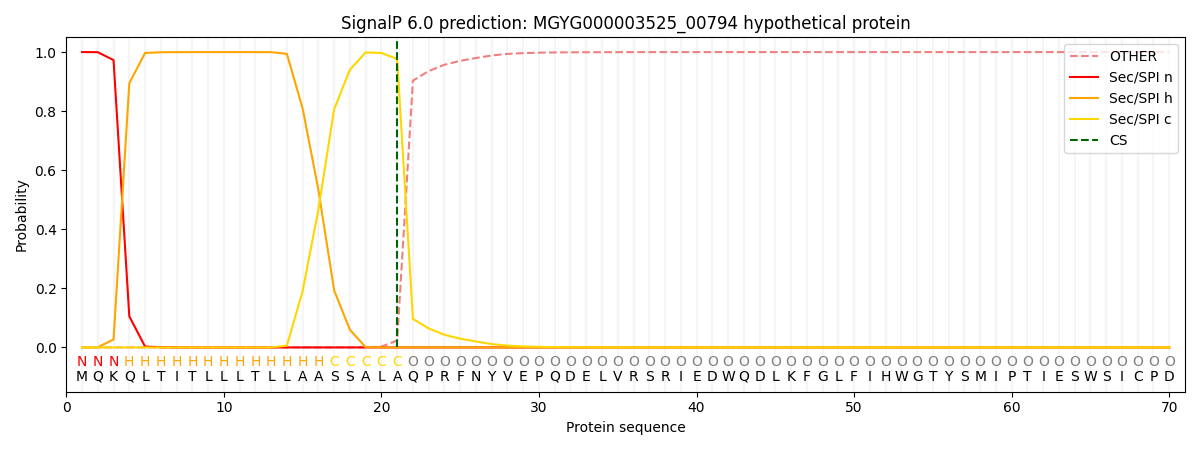
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000259 | 0.999129 | 0.000141 | 0.000183 | 0.000151 | 0.000144 |
