You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003638_01615
You are here: Home > Sequence: MGYG000003638_01615
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotellamassilia sp004557035 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotellamassilia; Prevotellamassilia sp004557035 | |||||||||||
| CAZyme ID | MGYG000003638_01615 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1461; End: 2375 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 35 | 132 | 6.7e-16 | 0.9791666666666666 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam13704 | Glyco_tranf_2_4 | 1.38e-13 | 35 | 133 | 1 | 97 | Glycosyl transferase family 2. Members of this family of prokaryotic proteins include putative glucosyltransferases, |
| cd02511 | Beta4Glucosyltransferase | 5.61e-08 | 32 | 233 | 6 | 176 | UDP-glucose LOS-beta-1,4 glucosyltransferase is required for biosynthesis of lipooligosaccharide. UDP-glucose: lipooligosaccharide (LOS) beta-1-4-glucosyltransferase catalyzes the addition of the first residue, glucose, of the lacto-N-neotetrase structure to HepI of the LOS inner core. LOS is the major constituent of the outer leaflet of the outer membrane of gram-positive bacteria. It consists of a short oligosaccharide chain of variable composition (alpha chain) attached to a branched inner core which is lined in turn to lipid A. Beta 1,4 glucosyltransferase is required to attach the alpha chain to the inner core. |
| pfam01697 | Glyco_transf_92 | 1.47e-06 | 39 | 224 | 18 | 208 | Glycosyltransferase family 92. Members of this family act as galactosyltransferases, belonging to glycosyltransferase family 92. The aligned region contains several conserved cysteine residues and several charged residues that may be catalytic residues. This is supported by the inclusion of this family in the GT-A glycosyl transferase superfamily. |
| cd00761 | Glyco_tranf_GTA_type | 0.001 | 33 | 126 | 4 | 93 | Glycosyltransferase family A (GT-A) includes diverse families of glycosyl transferases with a common GT-A type structural fold. Glycosyltransferases (GTs) are enzymes that synthesize oligosaccharides, polysaccharides, and glycoconjugates by transferring the sugar moiety from an activated nucleotide-sugar donor to an acceptor molecule, which may be a growing oligosaccharide, a lipid, or a protein. Based on the stereochemistry of the donor and acceptor molecules, GTs are classified as either retaining or inverting enzymes. To date, all GT structures adopt one of two possible folds, termed GT-A fold and GT-B fold. This hierarchy includes diverse families of glycosyl transferases with a common GT-A type structural fold, which has two tightly associated beta/alpha/beta domains that tend to form a continuous central sheet of at least eight beta-strands. The majority of the proteins in this superfamily are Glycosyltransferase family 2 (GT-2) proteins. But it also includes families GT-43, GT-6, GT-8, GT13 and GT-7; which are evolutionarily related to GT-2 and share structure similarities. |
| cd04179 | DPM_DPG-synthase_like | 0.001 | 36 | 119 | 7 | 89 | DPM_DPG-synthase_like is a member of the Glycosyltransferase 2 superfamily. DPM1 is the catalytic subunit of eukaryotic dolichol-phosphate mannose (DPM) synthase. DPM synthase is required for synthesis of the glycosylphosphatidylinositol (GPI) anchor, N-glycan precursor, protein O-mannose, and C-mannose. In higher eukaryotes,the enzyme has three subunits, DPM1, DPM2 and DPM3. DPM is synthesized from dolichol phosphate and GDP-Man on the cytosolic surface of the ER membrane by DPM synthase and then is flipped onto the luminal side and used as a donor substrate. In lower eukaryotes, such as Saccharomyces cerevisiae and Trypanosoma brucei, DPM synthase consists of a single component (Dpm1p and TbDpm1, respectively) that possesses one predicted transmembrane region near the C terminus for anchoring to the ER membrane. In contrast, the Dpm1 homologues of higher eukaryotes, namely fission yeast, fungi, and animals, have no transmembrane region, suggesting the existence of adapter molecules for membrane anchoring. This family also includes bacteria and archaea DPM1_like enzymes. However, the enzyme structure and mechanism of function are not well understood. The UDP-glucose:dolichyl-phosphate glucosyltransferase (DPG_synthase) is a transmembrane-bound enzyme of the endoplasmic reticulum involved in protein N-linked glycosylation. This enzyme catalyzes the transfer of glucose from UDP-glucose to dolichyl phosphate. This protein family belongs to Glycosyltransferase 2 superfamily. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QRQ49132.1 | 5.25e-140 | 5 | 297 | 6 | 298 |
| QEW34763.1 | 2.06e-139 | 1 | 297 | 1 | 297 |
| QUT86533.1 | 3.86e-139 | 1 | 297 | 1 | 297 |
| ALK83397.1 | 4.14e-139 | 1 | 297 | 1 | 297 |
| ABR38758.1 | 4.14e-139 | 1 | 297 | 1 | 297 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as OTHER
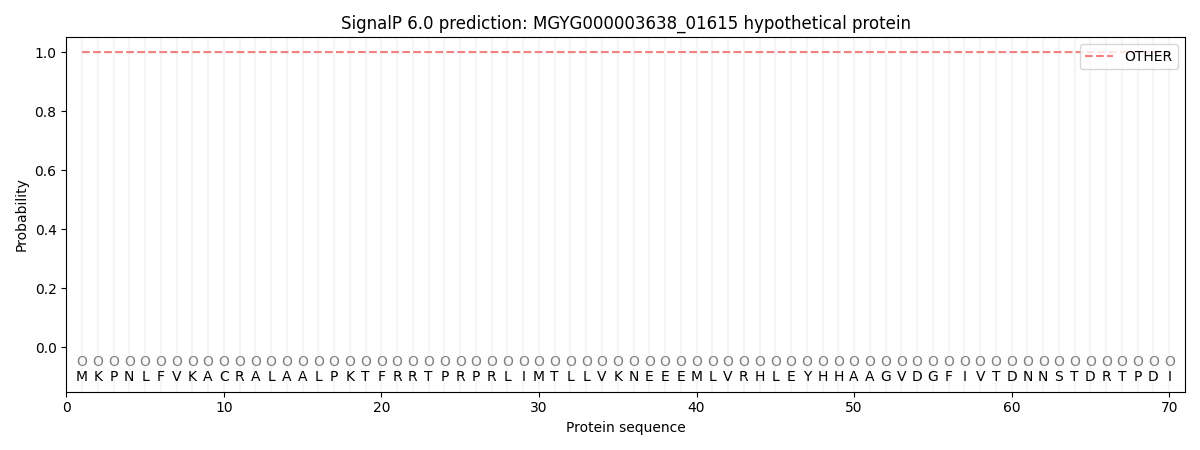
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000058 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |
