You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000003893_01243
You are here: Home > Sequence: MGYG000003893_01243
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella multisaccharivorax | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella multisaccharivorax | |||||||||||
| CAZyme ID | MGYG000003893_01243 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 20690; End: 22762 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH43 | 25 | 309 | 4.1e-105 | 0.9966555183946488 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd09019 | galactose_mutarotase_like | 7.04e-162 | 352 | 687 | 1 | 326 | galactose mutarotase_like. Galactose mutarotase catalyzes the conversion of beta-D-galactose to alpha-D-galactose. Beta-D-galactose is produced by the degradation of lactose, a disaccharide composed of beta-D-glucose and beta-D-galactose. This epimerization reaction is the first step in the four-step Leloir pathway, which converts galactose into metabolically important glucose. This epimerization step is followed by the phosophorylation of alpha-D-galactose by galactokinase, an enzyme which can only act on the alpha anomer. A glutamate and a histidine residue of the galactose mutarotase have been shown to be critical for catalysis, the glutamate serves as the active site base to initiate the reaction by removing the proton from the C-1 hydroxyl group of the sugar substrate, and the histidine as the active site acid to protonate the C-5 ring oxygen. Galactose mutarotase is a member of the aldose-1-epimerase superfamily. |
| cd18616 | GH43_ABN-like | 5.86e-140 | 27 | 304 | 1 | 291 | Glycosyl hydrolase family 43 such as arabinan endo-1 5-alpha-L-arabinosidase. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes with endo-alpha-L-arabinanase (ABN; EC 3.2.1.99) activity. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. The GH43 ABN enzymes hydrolyze alpha-1,5-L-arabinofuranoside linkages. These arabinan-degrading enzymes are important in the food industry for efficient production of L-arabinose from agricultural waste; L-arabinose is often used as a bioactive sweetener. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| TIGR02636 | galM_Leloir | 6.45e-110 | 347 | 688 | 1 | 336 | galactose mutarotase. Members of this protein family act as galactose mutarotase (D-galactose 1-epimerase) and participate in the Leloir pathway for galactose/glucose interconversion. All members of the seed alignment for this model are found in gene clusters with other enzymes of the Leloir pathway. This enzyme family belongs to the aldose 1-epimerase family, described by pfam01263. However, the enzyme described as aldose 1-epimerase itself (EC 5.1.3.3) is called broadly specific for D-glucose, L-arabinose, D-xylose, D-galactose, maltose and lactose. The restricted genome context for genes in this family suggests members should act primarily on D-galactose. |
| PRK11055 | galM | 1.48e-105 | 347 | 689 | 6 | 341 | galactose-1-epimerase; Provisional |
| PLN00194 | PLN00194 | 5.44e-87 | 358 | 689 | 15 | 337 | aldose 1-epimerase; Provisional |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUB89410.1 | 0.0 | 1 | 690 | 1 | 690 |
| AEA20065.1 | 0.0 | 1 | 690 | 1 | 690 |
| QUB93993.1 | 0.0 | 1 | 690 | 1 | 690 |
| QUB89901.1 | 0.0 | 1 | 690 | 1 | 690 |
| QUI92994.1 | 0.0 | 1 | 690 | 1 | 690 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1SNZ_A | 1.96e-71 | 361 | 688 | 31 | 342 | Crystalstructure of apo human galactose mutarotase [Homo sapiens],1SNZ_B Crystal structure of apo human galactose mutarotase [Homo sapiens],1SO0_A Crystal structure of human galactose mutarotase complexed with galactose [Homo sapiens],1SO0_B Crystal structure of human galactose mutarotase complexed with galactose [Homo sapiens],1SO0_C Crystal structure of human galactose mutarotase complexed with galactose [Homo sapiens],1SO0_D Crystal structure of human galactose mutarotase complexed with galactose [Homo sapiens] |
| 4RNL_A | 6.78e-61 | 347 | 690 | 19 | 343 | Thecrystal structure of a possible galactose mutarotase from Streptomyces platensis subsp. rosaceus [Streptomyces platensis],4RNL_B The crystal structure of a possible galactose mutarotase from Streptomyces platensis subsp. rosaceus [Streptomyces platensis],4RNL_C The crystal structure of a possible galactose mutarotase from Streptomyces platensis subsp. rosaceus [Streptomyces platensis],4RNL_D The crystal structure of a possible galactose mutarotase from Streptomyces platensis subsp. rosaceus [Streptomyces platensis] |
| 1L7J_A | 4.70e-44 | 347 | 690 | 9 | 339 | ChainA, galactose mutarotase [Lactococcus lactis],1L7J_B Chain B, galactose mutarotase [Lactococcus lactis],1L7K_A Chain A, galactose mutarotase [Lactococcus lactis],1L7K_B Chain B, galactose mutarotase [Lactococcus lactis],1MMU_A Chain A, Aldose 1-epimerase [Lactococcus lactis],1MMU_B Chain B, Aldose 1-epimerase [Lactococcus lactis],1MMX_A Chain A, Aldose 1-epimerase [Lactococcus lactis],1MMX_B Chain B, Aldose 1-epimerase [Lactococcus lactis],1MMY_A Chain A, Aldose 1-epimerase [Lactococcus lactis],1MMY_B Chain B, Aldose 1-epimerase [Lactococcus lactis],1MMZ_A Chain A, Aldose 1-epimerase [Lactococcus lactis],1MMZ_B Chain B, Aldose 1-epimerase [Lactococcus lactis],1MN0_A Chain A, Aldose 1-epimerase [Lactococcus lactis],1MN0_B Chain B, Aldose 1-epimerase [Lactococcus lactis] |
| 1NS0_A | 1.22e-43 | 347 | 690 | 9 | 339 | ChainA, GALACTOSE MUTAROTASE [Lactococcus lactis],1NS0_B Chain B, GALACTOSE MUTAROTASE [Lactococcus lactis],1NS4_A Chain A, GALACTOSE MUTAROTASE [Lactococcus lactis],1NS4_B Chain B, GALACTOSE MUTAROTASE [Lactococcus lactis] |
| 1LUR_A | 1.39e-43 | 355 | 688 | 17 | 336 | CrystalStructure of the GalM/aldose Epimerase Homologue from C. elegans, Northeast Structural Genomics Target WR66 [Caenorhabditis elegans],1LUR_B Crystal Structure of the GalM/aldose Epimerase Homologue from C. elegans, Northeast Structural Genomics Target WR66 [Caenorhabditis elegans] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8K157 | 1.63e-74 | 361 | 688 | 29 | 340 | Galactose mutarotase OS=Mus musculus OX=10090 GN=Galm PE=1 SV=1 |
| Q66HG4 | 4.94e-72 | 361 | 688 | 29 | 340 | Galactose mutarotase OS=Rattus norvegicus OX=10116 GN=Galm PE=1 SV=1 |
| P05149 | 7.91e-72 | 336 | 690 | 23 | 380 | Aldose 1-epimerase OS=Acinetobacter calcoaceticus OX=471 GN=mro PE=1 SV=1 |
| Q96C23 | 1.01e-70 | 361 | 688 | 29 | 340 | Galactose mutarotase OS=Homo sapiens OX=9606 GN=GALM PE=1 SV=1 |
| Q5R8U1 | 2.05e-69 | 361 | 688 | 29 | 340 | Galactose mutarotase OS=Pongo abelii OX=9601 GN=GALM PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
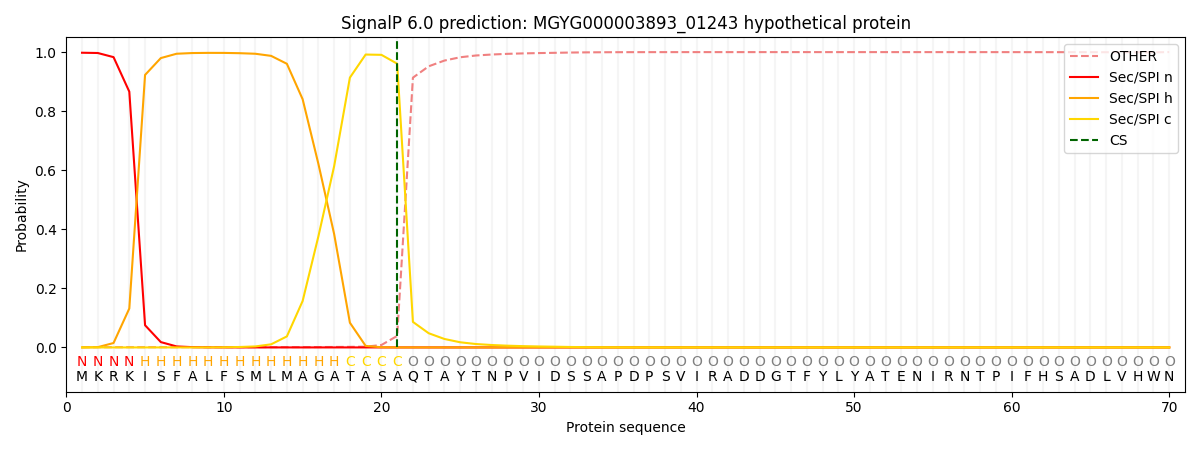
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002399 | 0.994835 | 0.001685 | 0.000604 | 0.000255 | 0.000211 |
