You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004123_00643
You are here: Home > Sequence: MGYG000004123_00643
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
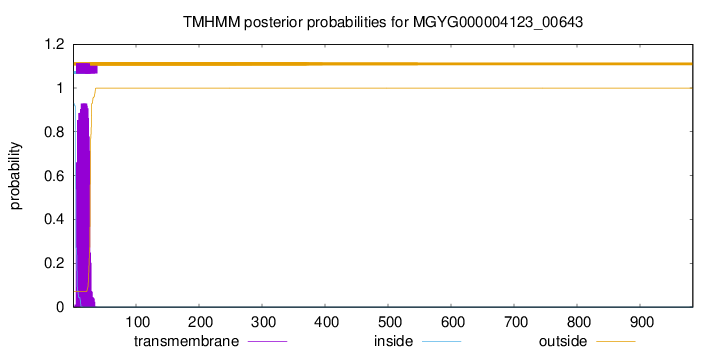
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Peptostreptococcales; Anaerovoracaceae; QAKD01; | |||||||||||
| CAZyme ID | MGYG000004123_00643 | |||||||||||
| CAZy Family | SLH | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 90737; End: 93691 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| SLH | 35 | 75 | 2.7e-18 | 0.9761904761904762 |
| SLH | 93 | 134 | 7.6e-16 | 0.9761904761904762 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam00395 | SLH | 7.48e-13 | 35 | 75 | 1 | 42 | S-layer homology domain. |
| pfam00395 | SLH | 2.28e-11 | 93 | 134 | 1 | 42 | S-layer homology domain. |
| pfam13205 | Big_5 | 2.22e-10 | 551 | 663 | 8 | 106 | Bacterial Ig-like domain. |
| NF033190 | inl_like_NEAT_1 | 5.60e-09 | 35 | 206 | 582 | 754 | NEAT domain-containing leucine-rich repeat protein. Members of this family have an N-terminal NEAT (near transporter) domain often associated with iron transport, followed by a leucine-rich repeat region with significant sequence similarity to the internalins of Listeria monocytogenes. However, since Bacillus cereus (from which this protein was described, in PMID:16978259) is not considered an intracellular pathogen, and the function may be iron transport rather than internalization, applying the name "internalin" to this family probably would be misleading. |
| pfam12733 | Cadherin-like | 4.82e-06 | 794 | 880 | 2 | 85 | Cadherin-like beta sandwich domain. This domain is found in several bacterial, metazoan and chlorophyte algal proteins. A profile-profile comparison recovered the cadherin domain and a comparison of the predicted structure of this domain with the crystal structure of the cadherin showed a congruent seven stranded secondary structure. The domain is widespread in bacteria and seen in the firmicutes, actinobacteria, certain proteobacteria, bacteroides and chlamydiae with an expansion in Clostridium. In contrast, it is limited in its distribution in eukaryotes suggesting that it was derived through lateral transfer from bacteria. In prokaryotes, this domain is widely fused to other domains such as FNIII (Fibronectin Type III), TIG, SLH (S-layer homology), discoidin, cell-wall-binding repeat domain and alpha-amylase-like glycohydrolases. These associations are suggestive of a carbohydrate-binding function for this cadherin-like domain. In animal proteins it is associated with an ATP-grasp domain. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QOX65054.1 | 3.69e-196 | 29 | 984 | 48 | 982 |
| VDN48011.1 | 2.60e-122 | 13 | 663 | 11 | 653 |
| QNL44990.1 | 2.38e-109 | 28 | 663 | 26 | 660 |
| VDN48012.1 | 3.41e-98 | 22 | 663 | 23 | 653 |
| QSZ27623.1 | 1.14e-57 | 35 | 426 | 46 | 454 |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| C6CRV0 | 2.48e-20 | 15 | 205 | 1260 | 1460 | Endo-1,4-beta-xylanase A OS=Paenibacillus sp. (strain JDR-2) OX=324057 GN=xynA1 PE=1 SV=1 |
| P38536 | 8.12e-18 | 23 | 206 | 1670 | 1857 | Amylopullulanase OS=Thermoanaerobacterium thermosulfurigenes OX=33950 GN=amyB PE=3 SV=2 |
| P19424 | 1.36e-17 | 9 | 206 | 15 | 216 | Endoglucanase OS=Bacillus sp. (strain KSM-635) OX=1415 PE=1 SV=1 |
| P38535 | 3.37e-17 | 17 | 206 | 890 | 1083 | Exoglucanase XynX OS=Acetivibrio thermocellus OX=1515 GN=xynX PE=3 SV=1 |
| Q06852 | 1.69e-16 | 30 | 164 | 2136 | 2285 | Cell surface glycoprotein 1 OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=olpB PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
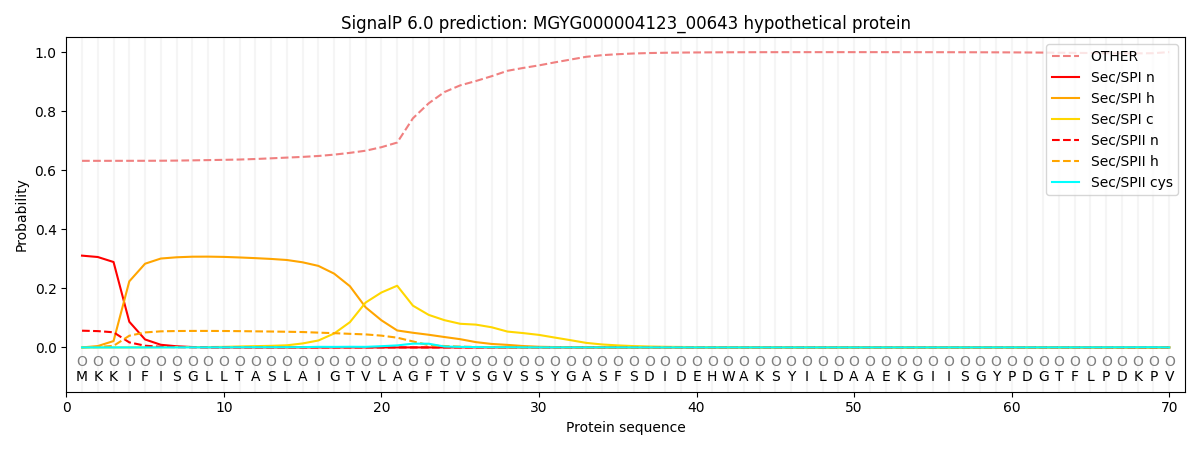
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.644882 | 0.293819 | 0.058306 | 0.000955 | 0.000547 | 0.001488 |