You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004128_00582
You are here: Home > Sequence: MGYG000004128_00582
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | UMGS1253 sp900550065 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Monoglobales_A; UMGS1253; UMGS1253; UMGS1253 sp900550065 | |||||||||||
| CAZyme ID | MGYG000004128_00582 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 19776; End: 20561 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 46 | 170 | 3.1e-30 | 0.9384615384615385 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| TIGR02764 | spore_ybaN_pdaB | 7.38e-58 | 48 | 238 | 1 | 191 | polysaccharide deacetylase family sporulation protein PdaB. This model describes the YbaN protein family, also called PdaB and SpoVIE, of Gram-positive bacteria. Although ybaN null mutants have only a mild sporulation defect, ybaN/ytrI double mutants show drastically reducted sporulation efficiencies. This synthetic defect suggests the role of this sigmaE-controlled gene in sporulation had been masked by functional redundancy. Members of this family are homologous to a characterized polysaccharide deacetylase; the exact function this protein family is unknown. [Cellular processes, Sporulation and germination] |
| cd10917 | CE4_NodB_like_6s_7s | 8.83e-57 | 53 | 205 | 1 | 153 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| cd10950 | CE4_BsYlxY_like | 4.21e-49 | 53 | 238 | 6 | 188 | Putative catalytic NodB homology domain of uncharacterized protein YlxY from Bacillus subtilis and its bacterial homologs. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. This family is represented by Bacillus subtilis putative polysaccharide deacetylase BsYlxY, encoded by the ylxY gene, which is a member of the carbohydrate esterase 4 (CE4) superfamily. Although its biological function still remains unknown, BsYlxY shows high sequence homology to the catalytic domain of Bacillus subtilis pdaB gene encoding a putative polysaccharide deacetylase (BsPdaB), which is essential for the maintenance of spores after the late stage of sporulation and is highly conserved in spore-forming bacteria. However, disruption of the ylxY gene in B. subtilis did not cause any sporulation defect. Moreover, the Asp residue in the classical His-His-Asp zinc-binding motif of CE4 esterases is mutated to a Val residue in this family. Other catalytically relevant residues of CE4 esterases are also not conserved, which suggest that members of this family may be inactive. |
| COG0726 | CDA1 | 9.08e-49 | 42 | 247 | 54 | 266 | Peptidoglycan/xylan/chitin deacetylase, PgdA/CDA1 family [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| cd10954 | CE4_CtAXE_like | 2.13e-46 | 53 | 237 | 1 | 180 | Catalytic NodB homology domain of Clostridium thermocellum acetylxylan esterase and its bacterial homologs. This family is represented by Clostridium thermocellum acetylxylan esterase (CtAXE, EC 3.1.1.72), a member of the carbohydrate esterase 4 (CE4) superfamily. CtAXE deacetylates O-acetylated xylan, a key component of plant cell walls. It shows no detectable activity on generic esterase substrates including para-nitrophenyl acetate. It is specific for sugar-based substrates and will precipitate acetylxylan, as a consequence of deacetylation. CtAXE is a monomeric protein containing a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold as other CE4 esterases. However, due to differences in the topography of the substrate-binding groove, the chemistry of the active center, and metal ion coordination, CtAXE has different metal ion preference and lacks activity on N-acetyl substrates. It is significantly activated by Co2+. Moreover, CtAXE displays distinctly different ligand coordination to the metal ion, utilizing an aspartate, a histidine, and four water molecules, as opposed to the conserved His-His-Asp zinc-binding triad of other CE4 esterases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADQ40949.1 | 8.55e-75 | 2 | 251 | 3 | 250 |
| AEM73484.1 | 2.43e-74 | 2 | 251 | 3 | 250 |
| ADQ06993.1 | 5.58e-73 | 2 | 251 | 3 | 250 |
| ADL42391.1 | 1.12e-72 | 2 | 251 | 3 | 250 |
| ACM60560.1 | 1.12e-72 | 2 | 251 | 3 | 250 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7FBW_A | 4.15e-30 | 53 | 240 | 117 | 302 | ChainA, Predicted xylanase/chitin deacetylase [Caldanaerobacter subterraneus subsp. tengcongensis MB4] |
| 6HM9_A | 1.91e-28 | 44 | 238 | 76 | 267 | Crystalstructure of a BA3943 mutant,a CE4 family pseudoenzyme with restored enzymatic activity. [Bacillus anthracis] |
| 2C1G_A | 1.03e-27 | 53 | 238 | 236 | 416 | Structureof Streptococcus pneumoniae peptidoglycan deacetylase (SpPgdA) [Streptococcus pneumoniae R6] |
| 6HPA_A | 2.51e-27 | 44 | 238 | 77 | 268 | Crystalstructure of a BA3943 mutant,a CE4 family pseudoenzyme [Bacillus anthracis] |
| 2C1I_A | 5.13e-27 | 53 | 238 | 236 | 416 | Structureof Streptococcus pneumoniae peptidoglycan deacetylase (SpPgdA) D 275 N Mutant. [Streptococcus pneumoniae R6] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8Y9V5 | 6.67e-30 | 39 | 237 | 246 | 445 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serovar 1/2a (strain ATCC BAA-679 / EGD-e) OX=169963 GN=pgdA PE=1 SV=1 |
| A0A0H3GDH9 | 6.67e-30 | 39 | 237 | 246 | 445 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serotype 1/2a (strain 10403S) OX=393133 GN=pgdA PE=2 SV=1 |
| A0A3Q0NBH7 | 6.67e-30 | 39 | 237 | 246 | 445 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serotype 1/2a (strain EGD / Mackaness) OX=1334565 GN=pgdA PE=1 SV=1 |
| Q8DP63 | 7.60e-27 | 53 | 238 | 268 | 448 | Peptidoglycan-N-acetylglucosamine deacetylase OS=Streptococcus pneumoniae (strain ATCC BAA-255 / R6) OX=171101 GN=pgdA PE=1 SV=1 |
| Q04729 | 3.19e-26 | 46 | 243 | 60 | 259 | Uncharacterized 30.6 kDa protein in fumA 3'region OS=Geobacillus stearothermophilus OX=1422 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
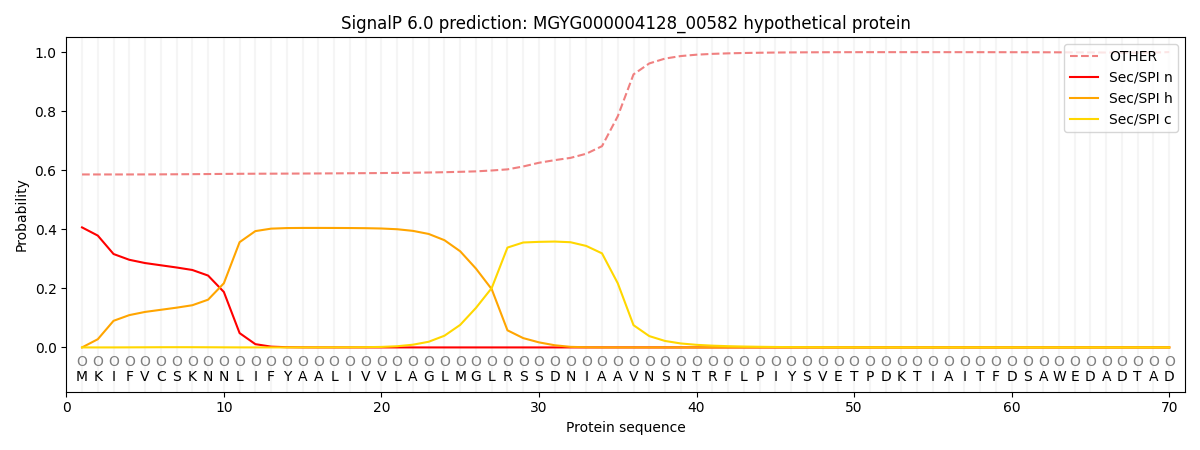
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.596805 | 0.392883 | 0.008841 | 0.000399 | 0.000254 | 0.000818 |

