You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004433_01714
You are here: Home > Sequence: MGYG000004433_01714
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
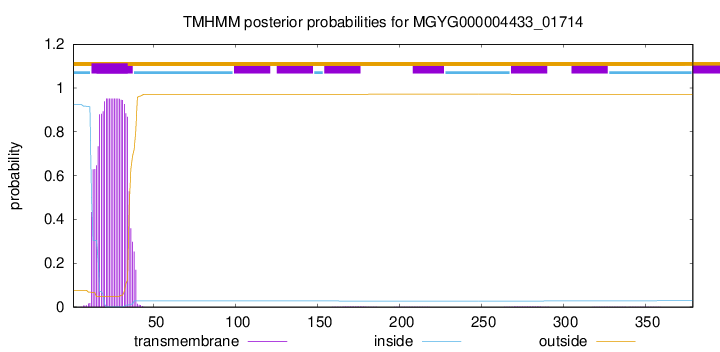
TMHMM annotations
Basic Information help
| Species | Bilophila sp900550745 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Desulfobacterota; Desulfovibrionia; Desulfovibrionales; Desulfovibrionaceae; Bilophila; Bilophila sp900550745 | |||||||||||
| CAZyme ID | MGYG000004433_01714 | |||||||||||
| CAZy Family | GH3 | |||||||||||
| CAZyme Description | Beta-hexosaminidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 3954; End: 5093 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH3 | 107 | 331 | 2.1e-54 | 0.9675925925925926 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG1472 | BglX | 5.55e-75 | 38 | 379 | 2 | 316 | Periplasmic beta-glucosidase and related glycosidases [Carbohydrate transport and metabolism]. |
| pfam00933 | Glyco_hydro_3 | 4.82e-63 | 38 | 374 | 1 | 316 | Glycosyl hydrolase family 3 N terminal domain. |
| PRK05337 | PRK05337 | 6.50e-51 | 72 | 370 | 27 | 308 | beta-hexosaminidase; Provisional |
| PRK15098 | PRK15098 | 4.98e-04 | 38 | 378 | 46 | 356 | beta-glucosidase BglX. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| EGB16456.1 | 1.49e-118 | 26 | 378 | 29 | 380 |
| EGJ48598.1 | 1.76e-117 | 42 | 378 | 40 | 375 |
| QGY41940.1 | 1.11e-115 | 42 | 375 | 1 | 334 |
| ABB39355.1 | 5.67e-115 | 32 | 375 | 29 | 378 |
| AAS96712.1 | 3.88e-114 | 24 | 375 | 39 | 391 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6K5J_A | 5.34e-59 | 38 | 377 | 12 | 339 | Structureof a glycoside hydrolase family 3 beta-N-acetylglucosaminidase from Paenibacillus sp. str. FPU-7 [Paenibacillaceae] |
| 3TEV_A | 2.03e-51 | 44 | 370 | 19 | 330 | Thecrystal structure of glycosyl hydrolase from Deinococcus radiodurans R1 [Deinococcus radiodurans R1],3TEV_B The crystal structure of glycosyl hydrolase from Deinococcus radiodurans R1 [Deinococcus radiodurans R1] |
| 4ZM6_A | 2.45e-48 | 38 | 377 | 8 | 339 | Aunique GCN5-related glucosamine N-acetyltransferase region exist in the fungal multi-domain GH3 beta-N-acetylglucosaminidase [Rhizomucor miehei CAU432],4ZM6_B A unique GCN5-related glucosamine N-acetyltransferase region exist in the fungal multi-domain GH3 beta-N-acetylglucosaminidase [Rhizomucor miehei CAU432] |
| 5BU9_A | 5.83e-46 | 38 | 375 | 6 | 337 | Crystalstructure of Beta-N-acetylhexosaminidase from Beutenbergia cavernae DSM 12333 [Beutenbergia cavernae DSM 12333],5BU9_B Crystal structure of Beta-N-acetylhexosaminidase from Beutenbergia cavernae DSM 12333 [Beutenbergia cavernae DSM 12333] |
| 3BMX_A | 9.27e-44 | 38 | 375 | 43 | 393 | Beta-N-hexosaminidase(YbbD) from Bacillus subtilis [Bacillus subtilis],3BMX_B Beta-N-hexosaminidase (YbbD) from Bacillus subtilis [Bacillus subtilis],3NVD_A Structure of YBBD in complex with pugnac [Bacillus subtilis],3NVD_B Structure of YBBD in complex with pugnac [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P40406 | 5.08e-43 | 38 | 375 | 43 | 393 | Beta-hexosaminidase OS=Bacillus subtilis (strain 168) OX=224308 GN=nagZ PE=1 SV=1 |
| Q5PGT0 | 5.53e-41 | 58 | 369 | 12 | 321 | Beta-hexosaminidase OS=Salmonella paratyphi A (strain ATCC 9150 / SARB42) OX=295319 GN=nagZ PE=3 SV=2 |
| B5RB93 | 5.53e-41 | 58 | 369 | 12 | 321 | Beta-hexosaminidase OS=Salmonella gallinarum (strain 287/91 / NCTC 13346) OX=550538 GN=nagZ PE=3 SV=1 |
| Q57QE6 | 5.53e-41 | 58 | 369 | 12 | 321 | Beta-hexosaminidase OS=Salmonella choleraesuis (strain SC-B67) OX=321314 GN=nagZ PE=3 SV=1 |
| A9N5J6 | 5.53e-41 | 58 | 369 | 12 | 321 | Beta-hexosaminidase OS=Salmonella paratyphi B (strain ATCC BAA-1250 / SPB7) OX=1016998 GN=nagZ PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
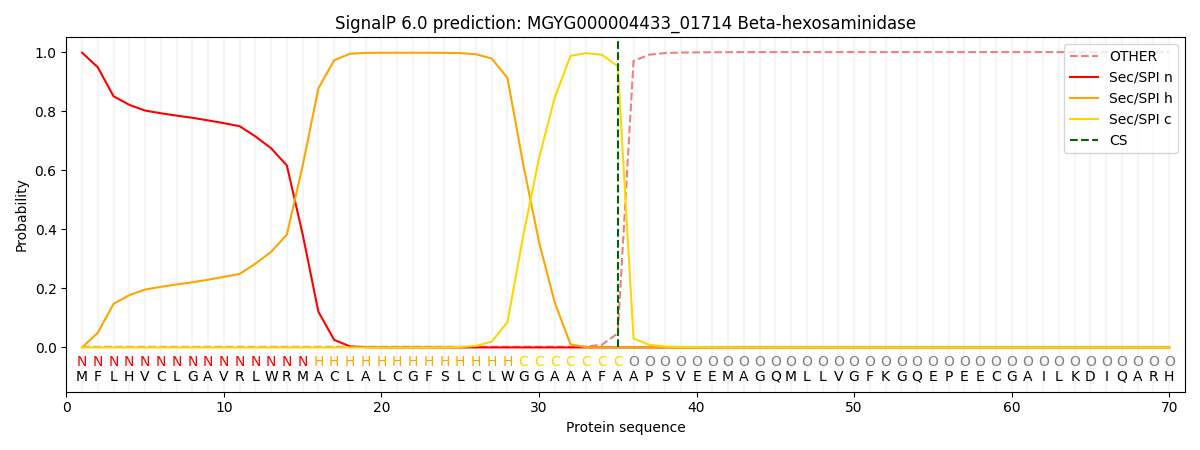
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.003230 | 0.995784 | 0.000323 | 0.000250 | 0.000192 | 0.000201 |