You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004570_00515
You are here: Home > Sequence: MGYG000004570_00515
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
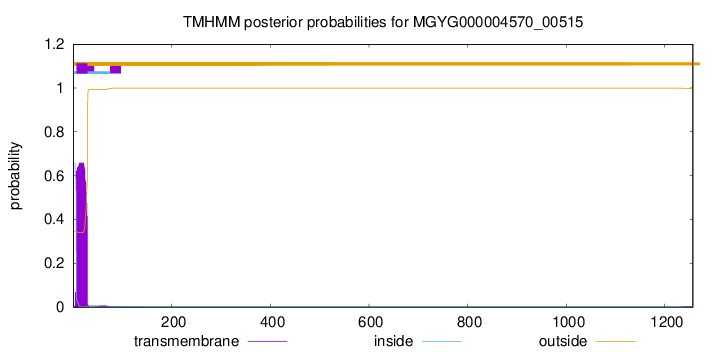
TMHMM annotations
Basic Information help
| Species | Treponema_D berlinense | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Spirochaetota; Spirochaetia; Treponematales; Treponemataceae; Treponema_D; Treponema_D berlinense | |||||||||||
| CAZyme ID | MGYG000004570_00515 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 185455; End: 189228 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL9 | 850 | 1235 | 1.1e-115 | 0.9466666666666667 |
| PL1 | 254 | 423 | 2.4e-46 | 0.8118811881188119 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3866 | PelB | 9.42e-50 | 239 | 542 | 82 | 340 | Pectate lyase [Carbohydrate transport and metabolism]. |
| smart00656 | Amb_all | 2.43e-30 | 253 | 425 | 14 | 190 | Amb_all domain. |
| pfam00544 | Pec_lyase_C | 5.76e-17 | 254 | 421 | 34 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
| pfam13229 | Beta_helix | 1.07e-06 | 929 | 1123 | 2 | 157 | Right handed beta helix region. This region contains a parallel beta helix region that shares some similarity with Pectate lyases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADL51369.1 | 2.33e-247 | 77 | 1248 | 60 | 1253 |
| AUS06374.1 | 2.28e-244 | 78 | 1131 | 26 | 1152 |
| QQY81127.1 | 1.86e-241 | 78 | 1131 | 26 | 1152 |
| AOR96287.1 | 4.69e-211 | 57 | 1249 | 38 | 1091 |
| QMW93302.1 | 1.00e-209 | 57 | 1249 | 38 | 1091 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1RU4_A | 3.35e-33 | 827 | 1134 | 4 | 298 | ChainA, Pectate lyase [Dickeya chrysanthemi] |
| 3VMV_A | 1.86e-22 | 257 | 423 | 81 | 248 | Crystalstructure of pectate lyase Bsp165PelA from Bacillus sp. N165 [Bacillus sp. N16-5],3VMW_A Crystal structure of pectate lyase Bsp165PelA from Bacillus sp. N165 in complex with trigalacturonate [Bacillus sp. N16-5] |
| 1VBL_A | 3.13e-17 | 258 | 421 | 135 | 330 | Structureof the thermostable pectate lyase PL 47 [Bacillus sp. TS-47] |
| 3ZSC_A | 3.80e-14 | 239 | 398 | 53 | 213 | Catalyticfunction and substrate recognition of the pectate lyase from Thermotoga maritima [Thermotoga maritima] |
| 5AMV_A | 1.99e-13 | 258 | 419 | 130 | 323 | Structuralinsights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P0C1A7 | 2.88e-32 | 827 | 1134 | 29 | 323 | Pectate lyase L OS=Dickeya dadantii (strain 3937) OX=198628 GN=pelL PE=1 SV=1 |
| P0C1A6 | 5.22e-32 | 851 | 1134 | 53 | 323 | Pectate lyase L OS=Dickeya chrysanthemi OX=556 GN=pelL PE=3 SV=1 |
| P22751 | 5.52e-27 | 861 | 1134 | 411 | 646 | Pectate disaccharide-lyase OS=Dickeya chrysanthemi OX=556 GN=pelX PE=1 SV=1 |
| Q65DC2 | 2.71e-26 | 199 | 429 | 60 | 280 | Pectate trisaccharide-lyase OS=Bacillus licheniformis (strain ATCC 14580 / DSM 13 / JCM 2505 / CCUG 7422 / NBRC 12200 / NCIMB 9375 / NCTC 10341 / NRRL NRS-1264 / Gibson 46) OX=279010 GN=BLi04129 PE=3 SV=1 |
| Q8GCB2 | 2.71e-26 | 199 | 429 | 60 | 280 | Pectate trisaccharide-lyase OS=Bacillus licheniformis OX=1402 GN=pelA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
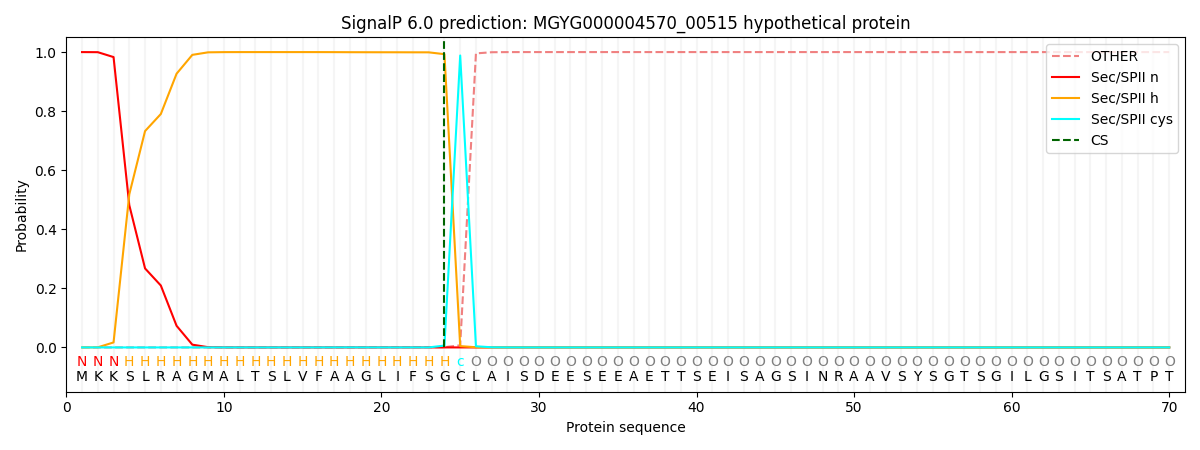
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000002 | 1.000064 | 0.000000 | 0.000000 | 0.000000 |