You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004737_01352
You are here: Home > Sequence: MGYG000004737_01352
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
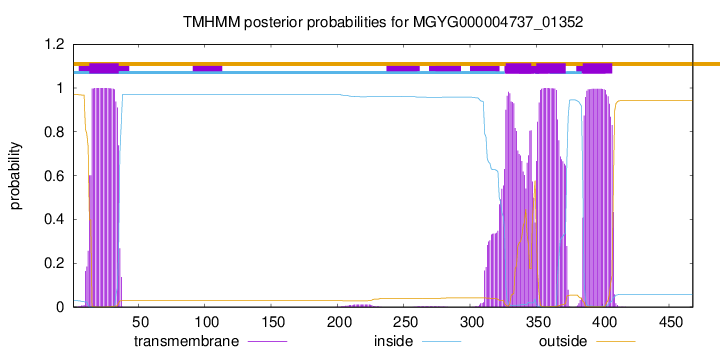
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Actinobacteriota; Coriobacteriia; Coriobacteriales; Atopobiaceae; Olsenella; | |||||||||||
| CAZyme ID | MGYG000004737_01352 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 33860; End: 35266 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 56 | 204 | 7e-23 | 0.8117647058823529 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| TIGR03111 | glyc2_xrt_Gpos1 | 1.20e-176 | 4 | 434 | 1 | 431 | putative glycosyltransferase, exosortase G-associated. Members of this protein family are probable glycosyltransferases of family 2, whose genes are near those for the exosortase homolog XrtG (TIGR03110), which is restricted to Gram-positive bacteria. Other genes in the conserved gene neighborhood include a 6-pyruvoyl tetrahydropterin synthase homolog (TIGR03112) and an uncharacterized intergral membrane protein (TIGR03766). |
| COG1215 | BcsA | 7.45e-49 | 31 | 435 | 32 | 437 | Glycosyltransferase, catalytic subunit of cellulose synthase and poly-beta-1,6-N-acetylglucosamine synthase [Cell motility]. |
| PRK11204 | PRK11204 | 1.52e-35 | 27 | 389 | 18 | 379 | N-glycosyltransferase; Provisional |
| cd06423 | CESA_like | 8.89e-34 | 57 | 245 | 1 | 180 | CESA_like is the cellulose synthase superfamily. The cellulose synthase (CESA) superfamily includes a wide variety of glycosyltransferase family 2 enzymes that share the common characteristic of catalyzing the elongation of polysaccharide chains. The members include cellulose synthase catalytic subunit, chitin synthase, glucan biosynthesis protein and other families of CESA-like proteins. Cellulose synthase catalyzes the polymerization reaction of cellulose, an aggregate of unbranched polymers of beta-1,4-linked glucose residues in plants, most algae, some bacteria and fungi, and even some animals. In bacteria, algae and lower eukaryotes, there is a second unrelated type of cellulose synthase (Type II), which produces acylated cellulose, a derivative of cellulose. Chitin synthase catalyzes the incorporation of GlcNAc from substrate UDP-GlcNAc into chitin, which is a linear homopolymer of beta-(1,4)-linked GlcNAc residues and Glucan Biosynthesis protein catalyzes the elongation of beta-1,2 polyglucose chains of Glucan. |
| PRK14583 | hmsR | 7.97e-25 | 33 | 417 | 52 | 432 | poly-beta-1,6 N-acetyl-D-glucosamine synthase. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ACR73592.1 | 1.14e-169 | 6 | 452 | 5 | 451 |
| QOX63067.1 | 4.71e-151 | 8 | 437 | 9 | 434 |
| BBF42945.1 | 1.57e-147 | 8 | 433 | 9 | 433 |
| QEY35692.1 | 1.08e-144 | 8 | 437 | 10 | 439 |
| ADK13482.1 | 2.10e-142 | 11 | 434 | 13 | 444 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6YV7_B | 6.01e-12 | 46 | 246 | 35 | 223 | MannosyltransferasePcManGT from Pyrobaculum calidifontis [Pyrobaculum calidifontis JCM 11548],6YV8_B Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP and Mn2+ [Pyrobaculum calidifontis JCM 11548],6YV9_A Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP-Man and Mn2+ [Pyrobaculum calidifontis JCM 11548] |
| 6YV7_A | 6.04e-12 | 46 | 246 | 36 | 224 | MannosyltransferasePcManGT from Pyrobaculum calidifontis [Pyrobaculum calidifontis JCM 11548],6YV8_A Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP and Mn2+ [Pyrobaculum calidifontis JCM 11548],6YV9_B Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP-Man and Mn2+ [Pyrobaculum calidifontis JCM 11548] |
| 5TZE_C | 1.49e-08 | 56 | 163 | 4 | 111 | Crystalstructure of S. aureus TarS in complex with UDP-GlcNAc [Staphylococcus aureus],5TZE_E Crystal structure of S. aureus TarS in complex with UDP-GlcNAc [Staphylococcus aureus],5TZI_C Crystal structure of S. aureus TarS 1-349 [Staphylococcus aureus],5TZJ_A Crystal structure of S. aureus TarS 1-349 in complex with UDP-GlcNAc [Staphylococcus aureus],5TZJ_C Crystal structure of S. aureus TarS 1-349 in complex with UDP-GlcNAc [Staphylococcus aureus],5TZK_C Crystal structure of S. aureus TarS 1-349 in complex with UDP [Staphylococcus aureus] |
| 5TZ8_A | 2.22e-08 | 56 | 163 | 4 | 111 | Crystalstructure of S. aureus TarS [Staphylococcus aureus],5TZ8_B Crystal structure of S. aureus TarS [Staphylococcus aureus],5TZ8_C Crystal structure of S. aureus TarS [Staphylococcus aureus] |
| 5HEA_A | 5.87e-08 | 55 | 158 | 7 | 107 | CgTstructure in hexamer [Streptococcus parasanguinis FW213],5HEA_B CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEA_C CgT structure in hexamer [Streptococcus parasanguinis FW213],5HEC_A CgT structure in dimer [Streptococcus parasanguinis FW213],5HEC_B CgT structure in dimer [Streptococcus parasanguinis FW213] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8XAR5 | 1.08e-22 | 30 | 316 | 50 | 331 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Escherichia coli O157:H7 OX=83334 GN=pgaC PE=3 SV=1 |
| P75905 | 6.52e-22 | 46 | 310 | 66 | 325 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Escherichia coli (strain K12) OX=83333 GN=pgaC PE=1 SV=1 |
| Q6GDD8 | 1.10e-19 | 55 | 336 | 49 | 311 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus aureus (strain MRSA252) OX=282458 GN=icaA PE=3 SV=1 |
| Q7A351 | 1.10e-19 | 55 | 336 | 49 | 311 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus aureus (strain N315) OX=158879 GN=icaA PE=3 SV=1 |
| Q9RQP9 | 1.10e-19 | 55 | 336 | 49 | 311 | Poly-beta-1,6-N-acetyl-D-glucosamine synthase OS=Staphylococcus aureus (strain NCTC 8325 / PS 47) OX=93061 GN=icaA PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000088 | 0.000001 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |