You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000004797_01020
You are here: Home > Sequence: MGYG000004797_01020
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Phocaeicola sartorii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Phocaeicola; Phocaeicola sartorii | |||||||||||
| CAZyme ID | MGYG000004797_01020 | |||||||||||
| CAZy Family | PL8 | |||||||||||
| CAZyme Description | Chondroitinase-AC | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 47571; End: 49604 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL8 | 350 | 596 | 2.2e-67 | 0.9919678714859438 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd01083 | GAG_Lyase | 2.09e-144 | 90 | 662 | 34 | 682 | Glycosaminoglycan (GAG) polysaccharide lyase family. This family consists of a group of secreted bacterial lyase enzymes capable of acting on glycosaminoglycans, such as hyaluronan and chondroitin, in the extracellular matrix of host tissues, contributing to the invasive capacity of the pathogen. These are broad-specificity glycosaminoglycan lyases which recognize uronyl residues in polysaccharides and cleave their glycosidic bonds via a beta-elimination reaction to form a double bond between C-4 and C-5 of the non-reducing terminal uronyl residues of released products. Substrates include chondroitin, chondroitin 4-sulfate, chondroitin 6-sulfate, and hyaluronic acid. Family members include chondroitin AC lyase, chondroitin abc lyase, xanthan lyase, and hyalurate lyase. |
| pfam02278 | Lyase_8 | 4.39e-89 | 350 | 598 | 1 | 252 | Polysaccharide lyase family 8, super-sandwich domain. This family consists of a group of secreted bacterial lyase enzymes EC:4.2.2.1 capable of acting on hyaluronan and chondroitin in the extracellular matrix of host tissues, contributing to the invasive capacity of the pathogen. |
| pfam08124 | Lyase_8_N | 1.01e-36 | 90 | 341 | 42 | 309 | Polysaccharide lyase family 8, N terminal alpha-helical domain. This family consists of a group of secreted bacterial lyase enzymes EC:4.2.2.1 capable of acting on hyaluronan and chondroitin in the extracellular matrix of host tissues, contributing to the invasive capacity of the pathogen. |
| cd17252 | RMtype1_S_EcoKI-TRD1-CR1_like | 0.005 | 446 | 528 | 6 | 90 | Type I restriction-modification system specificity (S) subunit Target Recognition Domain-ConseRved domain (TRD-CR), similar to S.EcoKI TRD1-CR1, S.StySPI TRD1-CR1, S.Ara36733II TRD1-CR1, and S.Eco3722I TRD1-CR1. Escherichia coli str. K-12 substr. MG1655 S subunit (S.EcoKI) and Escherichia coli NCM3722 S subunit (S.Eco3722I) recognize 5'... AACNNNNNNGTGC ... 3', Salmonella enterica subsp. enterica serovar Potsdam S subunit (S.StySPI) recognizes 5'... AACNNNNNNGTRC ... 3', and Actinomyces radicidentis S subunit (S.Ara36733II) recognizes 5'... CATCNNNNNNCTC ... 3'. The restriction-modification (RM) system S subunit consists of two variable target recognition domains (TRD1 and 2) and two conserved regions (CR1 and CR2) which separate the TRDs. The TRDs each bind to different specific sequences in the DNA. For example, S.EcoKI-TRD1 and S.StySPI-TRD1 recognize AAC/GTT, S.EcoKI-TRD2 recognizes GCAC/GTGC, and S.StySPI-TRD2 recognizes GYAC/GTRC. RM systems protect a bacterial cell against invasion of foreign DNA by endonucleolytic cleavage of DNA that lacks a site specific modification. The host genome is protected from cleavage by methylation of specific nucleotides in the target sites. In type I systems, both restriction and modification activities are present in one enzyme complex composed of one DNA specificity (S) subunit (this family), two modification (M) subunits and two restriction (R) subunits. This model contains both TRD1-CR1 and TRD2-CR2. It also includes TRD-CR-like sequence-recognition domains of various type II restriction enzymes and methyltransferases such as Treponema pedis T A4 putative Type IIG restriction enzyme/N6-adenine DNA methyltransferase RM.TpeTA4ORF2695P. It may also include type I DNA methyltransferases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QDO71119.1 | 1.76e-267 | 14 | 665 | 13 | 680 |
| ALJ58447.1 | 2.17e-263 | 14 | 665 | 13 | 680 |
| QUT90440.1 | 3.08e-263 | 14 | 665 | 13 | 680 |
| QUT47778.1 | 5.52e-248 | 19 | 657 | 18 | 672 |
| QCT78112.1 | 3.96e-221 | 22 | 657 | 19 | 654 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1CB8_A | 3.64e-76 | 77 | 606 | 23 | 574 | CHONDROITINASEAC LYASE FROM FLAVOBACTERIUM HEPARINUM [Pedobacter heparinus] |
| 1HM2_A | 5.66e-76 | 77 | 606 | 45 | 596 | ACTIVESITE OF CHONDROITINASE AC LYASE REVEALED BY THE STRUCTURE OF ENZYME-OLIGOSACCHARIDE COMPLEXES AND MUTAGENESIS [Pedobacter heparinus],1HM3_A Active Site Of Chondroitinase Ac Lyase Revealed By The Structure Of Enzyme-Oligosaccharide Complexes And Mutagenesis [Pedobacter heparinus],1HMU_A ACTIVE SITE OF CHONDROITINASE AC LYASE REVEALED BY THE STRUCTURE OF ENZYME-OLIGOSACCHARIDE COMPLEXES AND MUTAGENESIS [Pedobacter heparinus],1HMW_A Active Site Of Chondroitinase Ac Lyase Revealed By The Structure Of Enzyme-oligosaccharide Complexes And Mutagenesis [Pedobacter heparinus] |
| 1RWA_A | 1.12e-36 | 156 | 624 | 124 | 659 | Crystalstructure of Arthrobacter aurescens chondroitin AC lyase [Paenarthrobacter aurescens],1RWC_A Crystal structure of Arthrobacter aurescens chondroitin AC lyase [Paenarthrobacter aurescens],1RWF_A Crystal structure of Arthrobacter aurescens chondroitin AC lyase in complex with chondroitin tetrasaccharide [Paenarthrobacter aurescens],1RWG_A Crystal structure of Arthrobacter aurescens chondroitin AC lyase in complex with chondroitin tetrasaccharide [Paenarthrobacter aurescens],1RWH_A Crystal structure of Arthrobacter aurescens chondroitin AC lyase in complex with chondroitin tetrasaccharide [Paenarthrobacter aurescens] |
| 1RW9_A | 1.12e-36 | 156 | 624 | 124 | 659 | Crystalstructure of the Arthrobacter aurescens chondroitin AC lyase [Paenarthrobacter aurescens] |
| 2WCO_A | 2.06e-31 | 115 | 635 | 93 | 681 | Structuresof the Streptomyces coelicolor A3(2) Hyaluronan Lyase in Complex with Oligosaccharide Substrates and an Inhibitor [Streptomyces coelicolor A3(2)],2WDA_A The X-ray structure of the Streptomyces coelicolor A3 Chondroitin AC Lyase in Complex with Chondroitin sulphate [Streptomyces violaceoruber] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q59288 | 3.10e-75 | 77 | 606 | 45 | 596 | Chondroitinase-AC OS=Pedobacter heparinus (strain ATCC 13125 / DSM 2366 / CIP 104194 / JCM 7457 / NBRC 12017 / NCIMB 9290 / NRRL B-14731 / HIM 762-3) OX=485917 GN=cslA PE=1 SV=1 |
| Q9AQS0 | 4.55e-24 | 88 | 647 | 62 | 701 | Xanthan lyase OS=Bacillus sp. (strain GL1) OX=84635 GN=xly PE=1 SV=1 |
| Q59801 | 7.98e-22 | 156 | 617 | 182 | 713 | Hyaluronate lyase OS=Staphylococcus aureus (strain NCTC 8325 / PS 47) OX=93061 GN=hysA PE=3 SV=1 |
| Q53591 | 6.13e-20 | 94 | 660 | 304 | 960 | Hyaluronate lyase OS=Streptococcus agalactiae serotype III (strain NEM316) OX=211110 GN=hylB PE=1 SV=2 |
| P0CZ01 | 1.98e-15 | 113 | 624 | 108 | 694 | Hyaluronate lyase OS=Cutibacterium acnes (strain DSM 16379 / KPA171202) OX=267747 GN=PPA0380 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
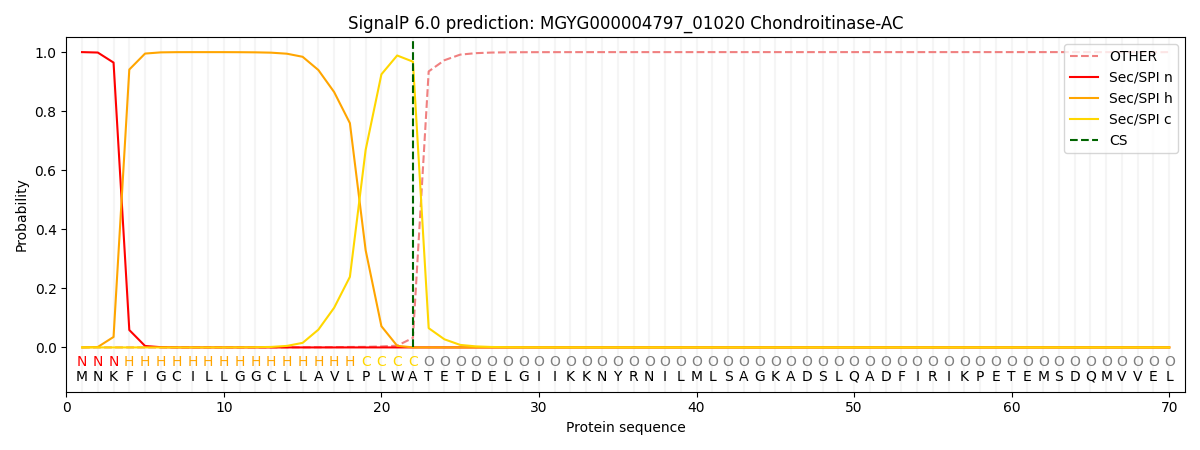
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.001305 | 0.997583 | 0.000408 | 0.000237 | 0.000233 | 0.000218 |
